Rare Earths- Lanthanides and Actinides
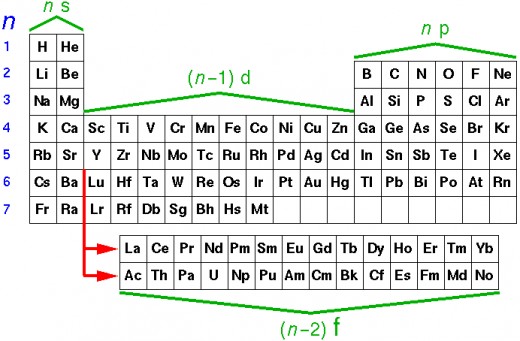
Elements in the periodic table are assigned to different blocks- s, p, d and f - blocks. Elements in which the differentiating electron or the last electron enters the f –orbital are called f-block elements.
The last electron or differentiating electron enters the pre-penultimate shell. Since the pre-penultimate shell is deep seated in nature therefore these elements are also call inner –transition elements. The f-block consists of two series of fourteen elements each placed at the bottom of the periodic table. The two series of the f-block elements are the lanthanides and the actinides. Lanthanides are the series of elements where the differentiating electron enters the 4f sub-shell. In the Actinides the differentiating electron enters the 5f sub-shell. The lanthanides and actinides are together also known as rare earths. Since the lanthanides are elements following lanthanum they are also called lanthanoids. The Actinoids are elements that follow actinium. The f block elements are placed in Group 3 of the periodic table, in two horizontal rows at the bottom of the periodic table. The lanthanides belong to the 6th period while the actinides belong to the 7th period. The outer three principal shells of the lanthanides and actinides are incompletely filled and so their general electronic configuration can be represented as (n-2)f 1-14 (n-1) d 0-1 ns2. For the lanthanides the general electronic configuration is [Xe] 54 4f1-14 5d1 6s2 while for the actinoids it is [Xe] 54 5f1-14 6d1 7s2. The general characteristics of f-block elements can be enumerated as follows-
1. Actinides and lanthanoids exhibit variable oxidation states.
2. They are metals with high melting points and high densities.
3. They form complexes that are colored.
4. Most of the elements of the actinoid series are radioactive in nature.
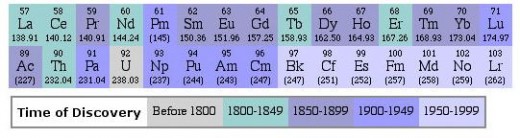
Lanthanides occur abundantly in nature. Each lanthanide mineral contains almost all the lanthanides. The lanthanide minerals can be classified into two groups-
Those minerals with atomic numbers 57-63 from Lanthanum to Europium (La to Eu ) are called cerite earth minerals.
The minerals containing largely yttrium and lanthanides from Gadolinium to Lutetium (Gd to Lu) are called yttrium earth minerals.
Some of the commercial ores of lanthanides are monazite, xenotime and bastnastite.