Redox Reactions

Definitions
The term redox is short for reduction-oxidation. Redox reactions are reactions that involve electron transfer. We can think of a redox reaction as consisting of two half reactions.
- Reduction = gain of electron(s)
- Oxidation = loss of electrons(s)
Here's a memory aid to remember the definition: LEO the lion says GER. Loss ofElectron is Oxidation, while Gain of Electron is Reducation. Another memory aid isOIL RIG. Oxidation Involves Loss of electron; ReducationInvolves Gain of electron. In a redox reaction, the reactant that is reduced is called the oxidizing agent while the reactant that is oxidized is the reducing agent.
Simple Redox Reactions
Example 1. Consider the reaction between copper metal, Cu(s), with an aqueous solution of silver nitrate, AgNO3(aq):
Cu(s) + 2 AgNO3(aq)→2 Ag(s) + Cu(NO3)2(aq)
In this reaction, a neutral Cu atom in Cu(s) ends up as copper(II) ion (Cu2+) in Cu(NO3)2. How does a neutral atom become positively charged? The answer is by losing electron(s). Therefore, we say that Cu atoms are oxidized to Cu2+ ions; we represent this half reaction by writing
Cu→Cu2+ + 2e-
Electrons are written as "products" of an oxidation half reaction. On the other hand, a silver(I) ion, Ag+, in AgNO3 ends up as a neutral Ag atom in Ag(s). A positive ion becomes a neutral atom by gaining electron(s). Therefore, we say that silver(I) ions are reduced to silver atoms in this reaction. We represent this half reaction by writing
Ag+ + e- →Ag
Electrons are written as "reactants" of a reduction half reaction.
Since Cu atoms in copper metal are oxidized, we say that the copper metal, Cu(s), is the reducing agent. Since Ag+ ions in AgNO3(aq) are reduced, we say that the silver nitrate solution is the oxidizing agent.
For videos of this reaction watch below:
The reaction of Cu with AgNO3 is an example of of a displacement reaction (also known as single replacement), a reaction that involves the displacement of an element from a compound by another element. In this case, we have Cu displacing Ag from AgNO3.
Any displacement reaction involves reduction and oxidation.
Whenever a metallic element is involved as a reactant, we can say that it is oxidized because atoms of a metallic element have a natural tendency to form positive ions (i.e., lose electrons). When a metallic element is produced in a reaction, we can say that it is the result of reduction of the metal cation.
Exercise: In a reaction where magnesium metal reacts to form a compound, magnesium is said to be A. oxidized, B. reduced, C. the oxidizing agent
Answer:
For videos of this reaction watch below:
Example 2: Consider the reaction between sodium metal and oxygen gas.
Mg(s) + O2(g) → 2 MgO(s)
We can identify Mg atoms having lost electrons. Mg atoms in elemental magnesium are neutral. In a compound, as in MgO, magnesium is always found as a cation with a charge of +2. We say that, in this case, magnesium is oxidized; oxidation involves a loss of electron. We represent the oxidation half reaction by writing
Mg → Mg2+ + 2e-
We can also identify the O atoms as having gained electrons. In MgO, oxygen exists as oxide ions (O2-). Since an O2 molecule is neutral, it appears that each O atom in the O2 molecule gained two electrons as they are converted to oxide ions in MgO. We say that the O atoms are reduced to oxide ions by gaining two electrons each.
Or we can say that each O2 molecule is reduced to two oxide ions by gaining four electrons. We represent this half reaction by writing
O2 + 4e- → 2 O2-
The formation of MgO from Mg and O2 is an example of a combination reaction (also known as synthesis or composition).
A combination reaction involves the formation of only one product from two or more reactants. Not all combination reactions are redox reactions. But if at least one of the reactants of a combination reaction is an element, then it is a redox reaction.
Examples of a combination reactions that do not involve reduction and oxidation:
- reaction of molecular base and acid to form a salt: NH3 + HCl→ NH4Cl
- reaction of metal hydroxide and carbon dioxide to form metal bicarbonate: LiOH + CO2 → LiHCO3
- reaction of metal oxide and water to form metal hydroxide: MgO + H2O → Mg(OH)2
The reaction in Example 2 occurs when pure Mg is exposed to air (which is about 20% O2); this reaction is similar to the rusting of iron, where Fe combines with O2to form rust (Fe2O3). This reaction can also happen really fast and release heat and light if Mg is ignited in a hot flame (i.e., burned); in this case the reaction is also classified as a combustion reaction. Combustion (burning) is a reaction with O2 that yields oxides of the elements and is accompanied with the production of heat and light. All combustion reactions are redox reactions.
For videos demonstrating these reactions watch below videos:
In a combustion reaction, the O atoms in O2 molecules are reduced and O2 is the oxidizing agent; the atoms of the substance being burned are oxidized.
Exercise: When Cl2 (g) reacts with a metallic element to form an ionic compound, we say that chlorine isA. oxidized, B. reduced, C. the reducing agent
Answer:
Example 3: Consider the reaction that occurs when HgO is heated:
2 HgO(s) → 2 Hg(l) + O2(g)
The mercury in HgO is a mercury(II) or Hg2+. These ions end up as neutral Hg atoms in liquid mercury, each ion gaining two electrons in the process. The oxide ions (O2-) in HgO end up as O2 molecules, losing a total of 4 electrons for each O2molecule formed. Thus, we say that Hg2+ ions are reduced and O2- ions are oxidized in this reaction. This reaction is an example of disproportionation, where the same substance (in this case, HgO) is oxidized and reduced.
For videos of this reaction watch below videos:
The grayish material formed on the sides of the test tube is liquid mercury. A glowing splint will ignite if inserted into the test tube; this is evidence for the production of O2.
The conversion of HgO to Hg and O2 is also classified as a decompositionreaction. A decomposition reaction involves the conversion of only one reactantinto two or more products. Think of decomposition as the opposite of combination (or synthesis or composition). Not all decomposition reactions are classified as redox reactions. But if one of the products of decomposition is an element, then we know that it is a redox reaction.
Examples of decomposition reactions that are not redox reactions:
- decomposition of metal carbonate to metal oxide and CO2: MgCO3 → MgO(s) + CO2
- decomposition of metal hydroxide to metal oxide and water: Mg(OH)2 → MgO(s) + H2O
- dehydration of a hydrate: CuSO4.5H2O → CuSO4 + 5 H2O
Exercise: When Br2(l) is produced from the reaction involving a bromine-containing binary ionic compound, the half reaction leading to the formation of Br2is classified asA. oxidation, B. reduction, C. oxidation or reduction depending on what the compound is
Answer:
For videos demonstrating this reaction watch below videos:
Example 4: Consider the combustion of carbon.
C(s) + O2(g) → CO2(g)
This reaction does not involve any ions, but is considered a redox reaction. Why? Even though C and O atoms are sharing electrons in CO2, the sharing is not equal. Shared electrons are, on average, actually spending more time closer to the O atom. We say that the covalent bond between C and O is polar, or that the electron density is polarized towards the O atom. We can say there is, in fact, a transfer of electrons from C to O, albeit a partial one.
Therefore, in this reaction, we say that C atoms "lost" electrons (oxidized), and that the O atoms "gained" electrons.
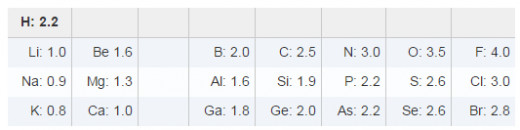
The ability of an atom to attract shared electrons is called electronegativity. We say that, in a polar bond, shared electrons are polarized towards them more electronegative atom. Therefore, we say that O is more electronegative than C. Linus Pauling assigned electronegativity values ranging from 0.7 for the least electronegative atom (Francium, bottom left corner of periodic table) to 4.0 for the most electronegative atom (Fluorine, near the top right corner). Some of Pauling's electronegativity values are given below. Note that electronegativity increases towards F. Metals have electronegativities less than 1.9 and nonmetals have electronegativities larger than 2.1.
Exercise: Which of the following chemical reactions is not a redox reaction?
A. C(s) + O2(g) → CO2(g)
B. Cl2(g) + 2 KBr(aq) → 2 KCl(aq) + Br2(l)
C. 2 KClO3(s) → 2 KCl(s) + 3 O2(g)
D. NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
Answer:
Exercise:Which of the following chemical reactions is a redox reaction?
A. NH3(g) + HCl (g)→ NH4Cl(s)
B. CaCO3(s) → CaO(s) + CO2(g)
C. 2 CaO(s)→ 2 Ca(s) + O2(g)
D. Ag+(aq) + Cl-(aq)→ AgCl(s)
Answer:
Oxidation Numbers
Keeping track of electrons lost and gained is useful for identifying and balancing redox reactions, but dealing with partial charges would be quite challenging and cumbersome. Therefore, as a matter of convenience, the concept ofoxidation number (or oxidation state) was invented. An atom is said to be oxidized if its oxidation number increases, and reduced if its oxidation number decreases.
The oxidation number of a monatomic species is its charge.
A monatomic species is either a neutral atom (with oxidation number defined as zero), or a monatomic ion. For atoms involved in covalent bonding (that is, in a molecule or polyatomic ion), charge is not defined; the entire species has a charge, but the atoms that make it up do not have a charge. In these cases, the oxidation number is defined as fictitious charge assigned to each atom assuming shared electrons "belong" to the more electronegative atom. Based on this, we can assign oxidation numbers by following these rules:
Rule 1. The oxidation number of atoms in an elemental substance is zero.
Example 1: What is the oxidation number of F atoms in F2?
Answer: In an F2 molecule, neither of the two F atoms is expected to attract shared electrons more strongly than the other; we expect equal sharing of electrons and neither atom can be thought of as having gained or lost an electron. The oxidation number of the fluorine atoms in the F2 molecule is zero.
Rule 2. In a compound,
- the oxidation number of a monatomic ion is equal to its charge
- the oxidation number of the most electronegative atomin a molecule or polyatomic ion is the charge it would have if it were to gain enough electrons to be isoelectronic with the closest noble gas; in other words:
- -1 if it belongs to group VIIA ( F, Cl, Br, I) or if it is H
- -2 if it belongs to group VIA (O, S, Se),
- -3 if it belongs to group VA (N, P),
- -4 if it belongs to group IVA (C)
- the sum of oxidation numbers of all atoms in a molecule is zero
- the sum of oxidation numbers of all atoms in a polyatomic ion is equal to the charge of the ion
Example 2: What is the oxidation number of chloride ion?
Answer: Chloride ion, Cl-, which is found in ionic compounds like NaCl and CaCl2, is a monatomic ion with a charge of -1. Therefore, the oxidation number is -1, the charge of monatomic ion.
Example 3: What are the oxidation numbers of Na and O in Na2O?
Answer: In Na2O, sodium exists as monatomic Na+ ions, while oxygen exists as oxide ions, O2-. Therefore, the oxidation numbers of Na and O in Na2O are +1 and -2.
Example 4: What are the oxidation numbers of B and H in BH3?
Answer: H is more electronegative than B. Therefore, we assign it an oxidation number of -1. If x is the oxidation number of B, then
x + 3(-1) = 0
since the oxidation numbers of atoms in a molecule add up to zero. Solving for x, we get x = +3. The oxidation of B in BH3 is +3.
Example 5: What are the oxidation numbers of O and F in OF2?
Answer: The oxidation number of F in any compound is -1 since it is the most electronegative atom. If the oxidation number of O is x, then
x + 2(-1) = 0
since the sum of all oxidation numbers must be equal to zero. Solving for x, we get x = +2.
Example 6: What are the oxidation numbers of K, N, and O in KNO3?
Answer: KNO3 is made up of potassium (K+) and nitrate (NO3-) ions. Therefore, the oxidation number of K is +1. O is more electronegative than N; therefore, in nitrate ion, its oxidation number is -2. If the oxidation number of N is x, then
x + 3(-2) = -1
since the oxidation numbers of N and the three O atoms should add up to the charge of nitrate, which is -1. Solving for x, we get x = +5.
Example 7: In a chemical reaction, the Cl in HCl molecules end up as Cl2molecules. Is Cl oxidized or reduced?
Answer: The oxidation number of Cl in HCl is -1 (since Cl is more electronegative than H). In Cl2 (an elemental substance), the oxidation number of Cl is 0. Therefore, Cl is oxidized; its oxidation number increases from -1 to 0.
Rule 2 should allow us to assign oxidation numbers to atoms in most compounds. However, there are exceptions. Sometimes, the best way to determine oxidation numbers is to draw the structure of the molecule or polyatomic ion. Oxidation numbers are determined in a manner similar to formal charges. The difference is that in determining formal charges, atoms sharing electrons are assigned equal ownership of the shared electrons regardless of electronegativities.
Exercise: Which of the following is false?
A. The charge of Hg in mercury(I) is +1.
B. The oxidation number of Hg in mercury(I) is +1
C. The charge of Hg in mercury(II) is +2
D. The oxidation number of Hg in mercury(II) is +2.
Answer:
Exercise: For which of the following is the oxidation number of F equal to zero?
A. CaF2
B. CF4
C. NaF
D. F2
Answer:
Exercise. Which of the following has the largest oxidation number for chlorine?
.A. MgCl2
B. CCl4
C. Cl2
D. ClO2-
Answer:

Balancing Redox Reactions
Chemical equations for redox reactions can be difficult to balance by inspection.
Example 1: Consider the following reaction, which is relatively easy to balance by inspection:
a HCl + b KMnO4 → c Cl2 + d MnCl2 + e KCl + f H2OUsing a, b, c, d, e, and f to represent coefficients, we can set up the following algebraic equations:
- Eq. 1, to balance H: a = 2f
- Eq. 2, to balance Cl: a = 2c + 2d + e
- Eq. 3, to balance K: b = e
- Eq. 4, to balance Mn: b = d
- Eq. 5, to balance O: 4b = f;
If an equation can be easily balanced by inspection, we should be able to assign any number to one of the coefficients, then figure out the others by making a series of simple substitutions. In this case, if we set a value to any one of the coefficients, except c, we can easily figure out all the others. For example, we can let a=1. Then:
- Substitute a=1 into Eq. 1: 1 = 2f; therefore: f = 1/2
- Substitute f=1/2 into Eq. 5: 4b = 1/2; therefore: b = 1/8
- Subsittute b=1/8 into Eq. 3: 1/8 = e; therefore: e = 1/8
- Substitute b=1/8 into Eq. 4: 1/8 = d; therefore: d = 1/8
- And substitute a=1, d=1/8, and e=1/8 into Eq. 2: 1 = 2c + 2(1/8) + 1/8; therefore: c = 5/16
Thus, a=1, b=1/8, c= 5/16, d=1/8, e=1/8, f=1/2 or, if we want the smallest set of whole number coefficients, we just multiply all the coefficients by 16 to get: a=16, b=2, c=5, d=2, e=2, and f=8, and our balanced equation is
16 HCl + 2 KMnO4 → 5 Cl2 + 2 MnCl2 + 2 KCl + 8 H2O
Example 2: Now consider the following redox reaction, which is somewhat harder to balance by inspection:
a H2S + b HNO3 c H2SO4 + d NO2 + e H2O
Using a, b, c, d, and e to represent coefficients, we can set up the following algebraic equations:
- Eq. 1, to balance H, Eq. 1: 2a + b = 2c + 2e
- Eq. 2, to balance S, Eq. 2: a = c
- Eq. 3, to balance N, Eq. 3: b = d
- Eq. 4, to balance O, Eq. 4: 3b = 4c + 2d + e
In this case, setting a value for e will not easily allow us to figure out the others. Setting a value for a, b, c, or d, will allow us to easily figure out one other coefficient, but we will be left with 3 simultaneous equations in 3 unknowns. For example, if we pick a=1, then c=1 using Eq. 2. To figure out the other variables, we will need to solve 3 equations (Eq. 1, 3, and 4) in 3 unknowns (b, d, and e). Here's how we could do it. Since, according to Eq. 3, b=3, we can eliminate b from Eq. 1 and 4:
- To balance H, Eq. 1 becomes: 2a + d = 2c + 2e, OR 2(1) + d = 2(1) + 2e, OR d = 2e
- To balance O, Eq. 2 becomes: 3d = 4c + 2d + e, OR 3d = 4(1) + 2d + e, OR d = 4 + e
Now, we have two equations in two unknowns (d and e). Substituting 2e for d in Eq. 2, we get
- 2e = 4 + e, OR e = 4
Substituting for e in Eq. 4, we get
- d = 2e = 2(4) = 8.
Finally, using Eq. 3,
- b = d = 8.
Thus, we have a=1, b=8, c=1, d=8, and e=4, and our balanced equation is
H2S + 8 HNO3 → H2SO4 + 8 NO2 + 4 H2O
We could simplify the balancing process if we add an additional constraint to our coefficients. This additional constraint is based on the fact that electrons lost must be equal to electrons gained.
Example 3: Consider the same reaction given in Example 2.
a H2S + b HNO3 → c H2SO4 + d NO2 + e H2O
By assigning oxidation numbers, we find that N is reduced and S is oxidized. The oxidation number of N is +5 in HNO3 and its oxidation number in NO2 is +4. A decrease in oxidation number by 1 means a gain of 1 electron for each N. Therefore, we can say that: 1 electron is gained for every NO2 produced.
The oxidation number of S in H2S is -2, and it is +6 in H2SO4. An increase in oxidation number by eight (from -2 to +6) means a loss of 8 electrons for each S. 8 electrons are lost for every H2SO4 molecule produced
Applying the constraint that electrons lost must equal electrons gained, we can say that the coefficients of NO2 and H2SO4 must be in an 8-to-1 ratio. Why? (8 molecules of NO2 ) times (1 electron gained per NO2 ) = 8 electrons gained (1 molecule of H2SO4) times (8 electrons lost per H2SO4) = 8 electrons lost Thus, we can simplify our problem by letting coefficient of NO2 = d = 8
coefficient of H2SO4 = c = 1
Substituting these into Eqs. 1-4 in Example 2, we get:
- Eq. 1, to balance H, Eq. 1: 2a + b = 2(1) + 2e
- Eq. 2, to balance S, Eq. 2: a = 1
- Eq. 3, to balance N, Eq. 3: b = 8
- Eq. 4, to balance O, Eq. 4: 3b = 4(1) + 2(8) + e
We can see right away from Eqs. 2 and 3 that a=1 and b=8. This leaves just one unknown to determine (e); we can solve for it using Eq. 1 or Eq. 4.
- Using Eq. 1: 2(1) + 8 = 2(1) + 2e, we find that e = 4
- Using Eq. 4: 3(8) = 4(1) + 2(8) + e, we also find that e = 4
The strategy described here will work as long as there is only one reduction product, only one oxidation product, and the reduction and oxidation products are not the same. If the reduction and oxidation product is just one, then try applying the electron-balance constraint on the coefficients of the reactants (oxidizing agent and reducing agent). Applying the electron-balance constraint to the reactants may not always work as in Example 1; in that particular case, not all the Cl in HCl ended up in Cl2; some ended in KCl and MnCl2 and are, thus, not being oxidized (same oxidation number, -1).
For redox reactions in aqueous solutions, it is preferable to write net ionic equations. When writing ionic equations, we write formulas of strong electrolytes in aqueous solution as separate ions. Then we can cancel spectator ions to get the net ionic equation.
Example 4: Write the net ionic equation for the redox reaction
H2S(aq) + 8 HNO3 (aq) → H2SO4 (aq) + 8 NO2 (g) + 4 H2O(l)
The strong electrolytes in aqueous solution in this reaction are HNO3 and H2SO4, which are two of the six known strong acids. Therefore, we rewrite the equation as
H2S(aq) + 8 H+(aq) + 8 NO3- (aq) → 2 H+(aq) + SO42-(aq) + 8 NO2 (g) + 4 H2O(l)
to get the full ionic equation. We can see that 2 H+(aq) is common to both sides, so the net ionic equation is
H2S(aq) + 6 H+(aq) + 8 NO3- (aq) → SO42-(aq) + 8 NO2 (g) + 4 H2O(l)
Example 5: Write the net ionic equation for the redox reaction
16 HCl(aq) + 2 KMnO4(s) → 5 Cl2(g) + 2 MnCl2 (aq) + 2 KCl(aq) + 8 H2O(l)
The strong electrolytes in aqueous solution in this reaction are HCl, MnCl2 and KCl. We write these formulas as separate ions. Note that KMnO4 is a strong electrolyte but the chemical equation suggests that solid KMnO4, not an aqueous solution of KMnO4, is being used as a reactant. Therefore, we do not write KMnO4as separate ions. The full ionic equation is
16 H+(aq) + 16 Cl-(aq) + 2 KMnO4(s) → 5 Cl2(g) + 2 Mn2+(aq) + 4Cl-(aq) + 2 K+(aq) + 2 Cl-(aq) + 8 H2O(l)
and the net ionic equation is obtained by cancelling the spectator ions. 6 of the 16 Cl- ions are spectator ions; only 10 are actually involved in the reaction. So, the net ionic equation is:
16 H+(aq) + 10 Cl-(aq) + 2 KMnO4(s) → 5 Cl2(g) + 2 Mn2+(aq) + 2 K+(aq) + 8 H2O(l)
For videos demonstrating this reaction watch below:
Example 6: Write the net ionic equation for the redox reaction
16 HCl(aq) + 2 KMnO4(aq) → 5 Cl2(g) + 2 MnCl2 (aq) + 2 KCl(aq) + 8 H2O(l)
This is similar to Example 5, except that KMnO4 is in aqueous solution. Since KMnO4 is an ionic compound, it is completely dissociated in aqueous solution; we need to write it as separate ions. The net result is that K+ ions need to be cancelled out as spectator ions.
16 H+(aq) + 10 Cl-(aq) + 2 MnO4-(aq) → 5 Cl2(g) + 2 Mn2+(aq) + 8 H2O(l)
Writing the net ionic equation is easy if you already have the balanced "molecular" equation as in Examples 5 and 6. However, if you have to start from an unbalanced equation, it is easier to just directly derive the net ionic equation using the ion-electron method. In this method, we identify the ions and molecules that contain the atoms that are actually oxidized or reduced and use those to construct half reactions. We balance the half reactions and combine them so that electrons lost equals electrons gained. To balance a half reaction in acidic solution we
- balance all atoms besides H and O
- balance O by adding H2O to the side that needs it
- balance H by adding H+ to the side that needs it
- balance charges by adding electrons to the more positive (or less negative) side
Example 7: Consider the following reaction in aqueous solution.
HCl(aq) + KMnO4 (aq) → Cl2 (g) + MnCl2(g) + KCl(aq) + H2O(l)
By assigning oxidation numbers, we find that Mn is reduced. Its oxidation number is +7 in KMnO4 and +2 in MnCl2. Since both KMnO4 and MnCl2 are ionic compounds, we expect them to be completely dissociated in aqueous solution. So the (unbalanced) half reaction for reduction will only have the ions that actually contain Mn.
MnO4- →Mn2+
Let's balance this half reaction.
- Balance all atoms besides H and O: MnO4- → Mn2+ (Mn is already balanced)
- Balance O: (need 4 more O atoms on the right side, so add 4 H2O to the right side)
MnO4- → Mn2+ + 4 H2O - Balance H: (need 8 H atoms on the left side, so add 8 H+ to the left side)
8 H+ + MnO4- → Mn2+ + 4 H2O - Balance charges.
Total charge on the left = 8(+1) + 1(-1) = +7.
Total charge on the right = 1(+2) + 4(0) = +2.
Add electrons to the left side, which is the more positive side. Add 5 electrons to the left side
so that the total charge on that side will equal the total charge on the right side:
5e- + 8 H+ + MnO4-→ Mn2+ + 4 H2O - Total charge on the left with 5 electrons included = 8(+1) + 1(-1) + 5(-1) = +2
Note that the number of electrons needed to balance charges is equal to the change in oxidation number; Mn is +7 in MnO4-, +2 in Mn2+.
Similarly, we find that Cl is oxidized. Its oxidation number in HCl is -1 and its oxidation number in Cl2 is 0. HCl is a strong acid and it is completely dissociated in aqueous solution. Cl2, on the other hand, is a molecular substance and in the gas phase. Thus, the (unbalanced) half reaction for oxidation is
Cl- → Cl2
Let's balance this half reaction.
- Balance all atoms besides H and O: 2 Cl- → Cl2
- Balance O: not necessary
- Balance H: not necessary
- Balance charges: add 2e- to the right side: 2 Cl- → Cl2 + 2e-
Finally we combine the two half reactions, keeping in mind that electrons lost must equal electrons gained. Since the reduction half reaction involves a gain of 5 electrons, and the oxidation half reaction involves a loss of 2 electrons, we can balance electrons lost and gained by multiplying the reduction half reaction by 2 and multiplying the oxidation half reaction by 5. The reduction half reaction, times 2,
2 ( 5e- + 8 H+ + MnO4- → Mn2+ + 4 H2O )
becomes: 10 e- + 16 H+ + 2 MnO4- → 2 Mn2+ + 8 H2O
and the oxidation half reaction, times 5,
5 ( 2 Cl- → Cl2 + 2e- )
becomes: 10 Cl- → 5 Cl2 + 10 e- Putting the two half reactions together, we get 16 H+(aq) + 10 Cl-(aq) + 2 MnO4-(aq) → 5 Cl2(g) + 2 Mn2+(aq) + 8 H2O(l)Note that the 10e- term, which appears on opposite sides in the half reactions, disappears in the net ionic equation.
When balancing half reactions in basic solution, we do one more step, replace H+by H2O, then add as many OH- ions to the other side. Then, cancel common terms.
Example 8: Write the corresponding net ionic equation if the following half reaction (balanced in acidic solution) were to occur in basic solution:
2 H2O(l) + SO2(g) → SO42-(aq) + 4 H+(aq) + 2e-
Replace 4 H+(aq) by 4 H2O(l), then add 4 OH-(aq) to the other side:
4 OH-(aq) + 2 H2O(l) + SO2(g) → SO42-(aq) + 4 H2O(l) + 2e-
Simplify by cancelling out common terms: subtract 2 H2O from both sides.
4 OH-(aq) + SO2(g) → SO42-(aq) + 2 H2O(l) + 2e-
Alternatively, we can balance H and O by doing the following:
- to the side with too much H, add OH-(aq), then add H2O(l) to the other side
- to the side with too much O, add H2O(l), then add 2 OH-(aq) to the other side
Example 9: Balance the following half reaction in basic solution.: MnO4-(aq) → MnO2(s)
- Balance all atoms besides H and O: Mn balanced as is
- Balance H: not necessary
- Balance O: add 2 H2O(l) to the left side since it has 2 more O compared to the right, then
add 4 OH-(aq) to the other side
2 H2O(l) + MnO4-(aq) → MnO2(s) + 4 OH-(aq) - Balance charges by adding electrons to the less negative side (3e- to the left side)
3e- + 2 H2O(l) + MnO4-(aq) → MnO2(s) + 4 OH-(aq)

Predicting Redox Reactions
Most commonly observed half reactions in aqueous solution are listed in a table ofstandard reduction potentials or standard electrode potentials. The half reactions in this table are written as reductions and the tabulated values (in Volts) associated with each half reaction give us an indication of the propensity for the half reaction to occur. The more positive the value of the standard reduction potential (Eo), the higher the propensity.
Example 1: Consider the following half reactions
Zn2+(aq) + 2e- → Zn(s), Eo = -0.76 V.
Cu2+(aq) + 2e- → Cu(s), Eo = +0.34 V
Which has a higher propensity to be reduced?
A. Zn2+, B. Zn, C. Cu2+, D. Cu
Answer: Cu2+ has a higher propensity to be reduced to Cu since its standard reduction potential is more positive.
Based on the information given in Example 1, we can also say that that Zn has a higher propensity to be oxidized to Zn2+.
Remember that the reverse of a reduction half reaction is oxidation. The standard oxidation potentials of Zn and Cu are just the negative of the standard reduction potentials. Zn(s) → Zn2+(aq) + 2e- Eo = +0.76 V.
Cu(s) → Cu2+(aq) + 2e- Eo = -0.34 V.
We can use this information to predict in Zn will displace Cu from its compound in solution. Zn has a greater tendency to get oxidized and go into solution as Zn2+ions. On the other hand, as we have seen in Example 1, Cu2+has a greater tendency to be reduced to Cu. In other words, the reaction Cu2+(aq) + Zn(s) Cu(s) + Zn2+(aq) has a greater tendency to occur compared to Zn2+(aq) + Cu(s) Zn(s) + Cu2+(aq)
We can use values of standard reduction potentials to construct the so-calledactivity series that we use to predict displacement reactions. The activity series for metals is actually a listing of the metals in order of decreasing standard oxidation potentials. The series for some of the most common metals goes as follows:
K Ca Na Mg Al Mn Zn Cr Fe Ni Sn Pb H Cu Bi Sb Hg Ag Pt Au
Note that Zn is higher than Cu in the activity series; it has a higher oxidation potential. A memory aid for this is: Peter Carly Simon Made A Mangy Zebra Carry Iron Nails To Liverpool. He Caught Billy, A Mexican Silver Plated Goat. Peter is for potassium (K), Carly is for calcium (Ca), etc.
The activity series for the halogens, F Cl Br I, is used to predict whether halogen can displace another from its compound. Fluorine (F2) can displace chlorine from compounds containing chloride ions. If we refer to a table of standard reduction potential, we find that the standard reduction potential of F2 is higher than that of Cl2.
F2 + 2e- → 2 F- , Eo = +2.87 V
Cl2 + 2e- → 2 Cl- , Eo = +1.36 V
You can find numerous internet sites with tables of standard reduction potentials:
References
© 2015 Discover the World