So what exactly is molecular polarity?
Alright, so at some point in everyone’s high school career, there comes a time when the idea of polarity is presented to the student. Now, thinking back to my own personal experience, I had a hard time grasping the most critical and fundamental aspects of the topic. Now, being a biochemistry major, I just look back and laugh. It really is a fairly simple matter.

So what exactly is it? Well, the most basic explanation is it’s the unevenly distributed charge on a molecule that allows it to be pulled or pushed away from other molecules. A fairly well observed water molecule, for instance, has this uneven charge. The oxygen in the central region of the figure below has a negative charge (I’ll explain why here shortly), and the two attached hydrogen molecules have positive charges.
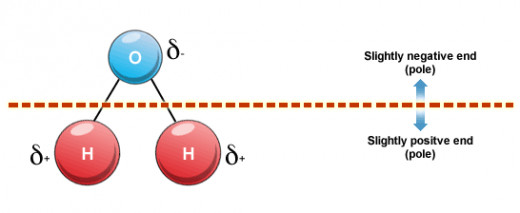
These react in a similar manner as magnets—essentially, atoms with positive charges will be strongly attracted to other atoms that have a negative charge, while atoms with like charges will tend to separate away from each other.
This allows for molecular organization that, quite frankly, plays a huge role in our lives and in almost all forms of matter. Think crystals. Polarity matters there because there is a repetitive pattern at the molecular level that is enabled most commonly by these charges and where on a molecule they are located.
As for the actual force, the easiest way, in my own opinion, to visualize it is to imagine something similar to gravity. Yes, there is a much more complex system when you enter the world of electrons, but for practical reasons, you may just want to understand that objects have attraction to others based on specific characteristics, much like density can affect gravity!
And keep in mind one of the things I mentioned earlier, that these charges must be unevenly distributed before the molecule can be considered polar. If you can draw a straight line down a center point in a basic Lewis structure (a structure with atoms and indicative bond lines as seen with water above) and see that one side has a different charge than the other, then there is reason to suggest that it is polar. It’s that simple!
Well… not quite.
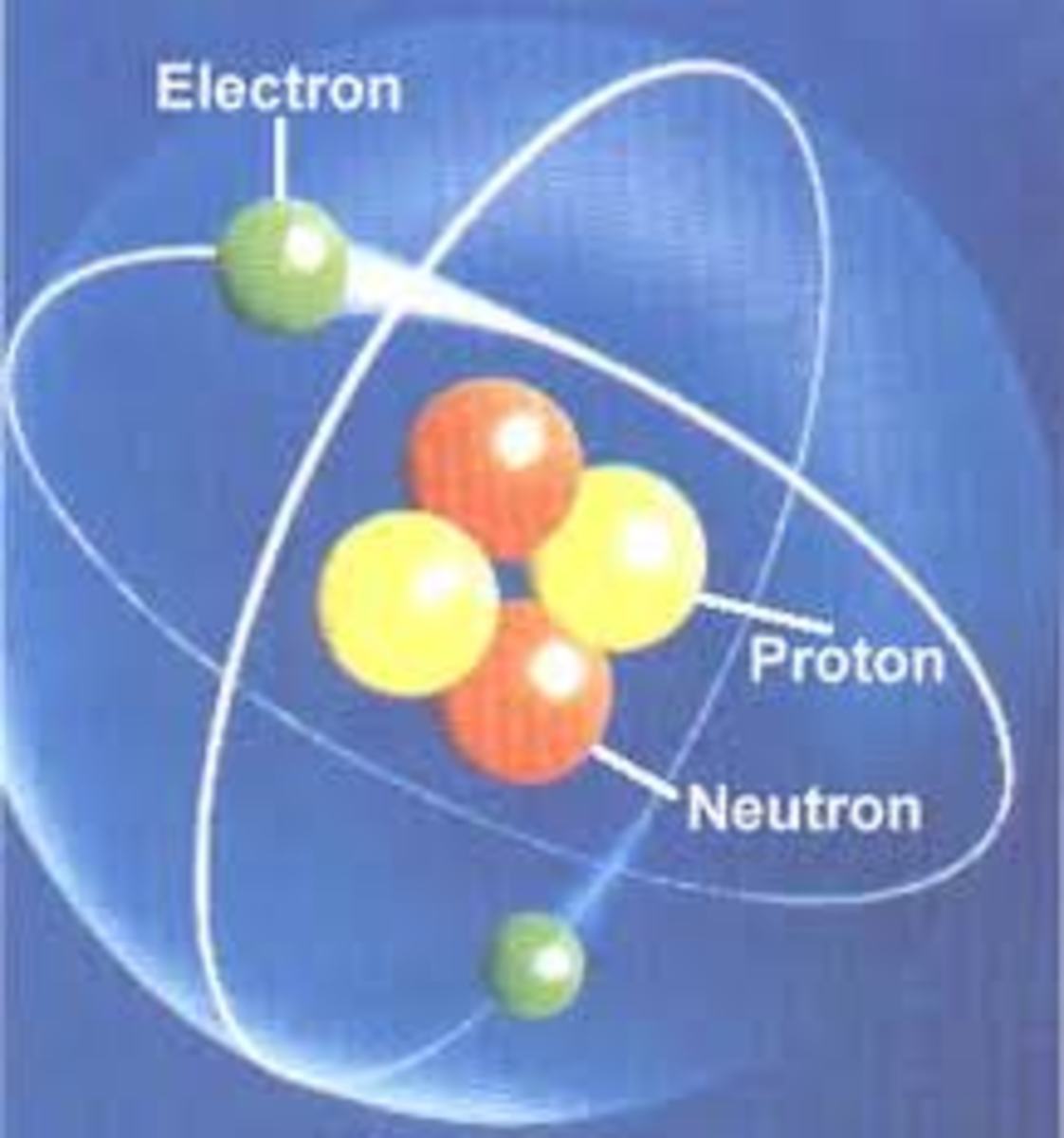
Lets take a little bit of a closer look at what is actually occurring at the atom level. As you most likely know, an atom consists of three major parts—protons and neutrons, which make up the nucleus and then the electrons, which create this sort of ‘cloud thingy’ around the nucleus. And it’s this electron cloud that affects charge. You may also know that, as protons have positive charges, electrons have negative ones. This is why the two stay together and what ultimately decides whether or not the end result will be considered ‘polar’. The electrons aren’t physically attached to the nucleus, but instead are flying around in the cloud, orbiting the nucleus like the moon goes around the earth (more advanced people can appreciate this futile thought).
Thankfully, we have a little thing called atomic bonding (the combination of two or more atoms to form a molecule). The reason this occurs is because different sized atoms, which have positive charges greater than others, can actually pull electrons away from others. That is to say, you can probably paint a picture in your mind of two guys having a tug-of-war match. The rope in this case is the electrons and there are forces pulling the rope in both directions, just like two interacting atoms will pull electrons toward themselves. But, as you’d expect, often one atom will be stronger than the other. The measure of an atoms ability to pull an electron from another atom is called its electron affinity. An atom that is ‘stronger’ and can beat out others and pull more electrons away from others is said to have a higher electronegativity. Below is a table of electronegativities for each element. Yes, each one has it’s own number, and those numbers really do matter.
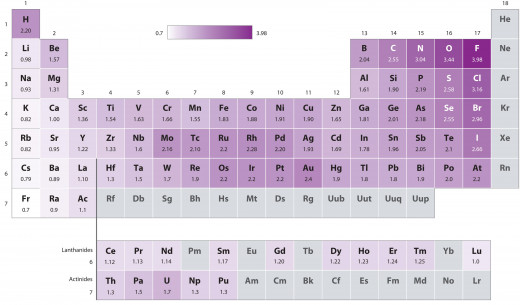
So what actually happens when two molecules have differing strengths? Well, one is going to win of course! The question is: where exactly does the rope go? See, when the two atoms interact, or bond together, the stronger atom can actually pull electrons away from the other atom and keep them all for itself! Or, maybe it can’t quite pull the electrons completely away, but far enough that the electrons are somewhere in the middle, where both atoms have to share them. The first is considered an ionic bond, and the second is considered a covalent one. But still, we haven’t answered the question. How can one part of a molecule be charged?
Think of it this way… And yes, I use a lot of similes. Get used to it. Normally, for every positively charged proton in an individual atom, there is a negatively charged electron to counter-balance the whole thing. A positive +1 and a negative -1 will cancel out and equal 0 charge. But, and maybe you can see where I am going with this, when an atom loses an electron to another atom, that match up wont occur! There will now be a positive charge on the atom where it lost its electron, because there’s a proton without its buddy to cancel it out! And the atom that took the electron has an extra negatively charged particle that it may not have had a proton for. So the electron taker will be negative and the electron loser will be positive.
Now you can draw that imaginary line and sometimes see that two sides of a molecule can have different charges that attract different things on each side!
Those are the basics of Polarity!
Want to know more? Well here’s a few links:
Electron configurations and octects:
http://www.khanacademy.org/science/chemistry/orbitals-and-electrons/v/electron-configurations
Acids and Bases:
http://www.khanacademy.org/science/chemistry/acids-and-bases
Bonding trends:
http://www.visionlearning.com/en/library/Chemistry/1/Chemical-Bonding/55