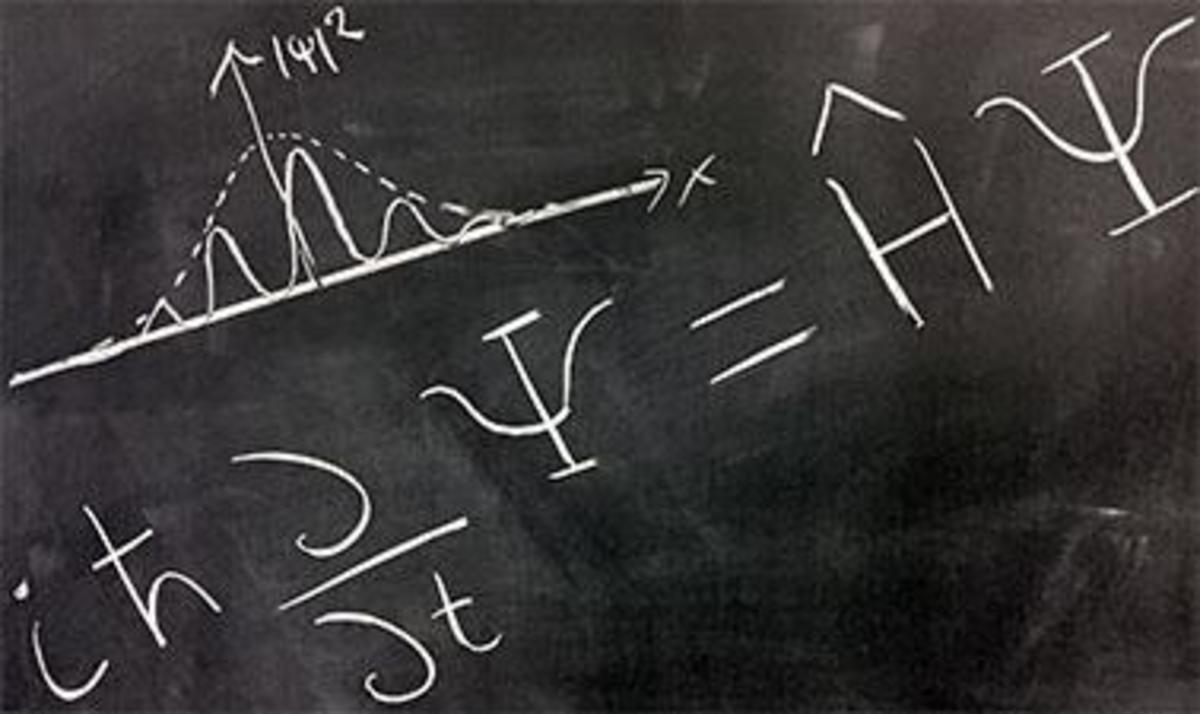Simple Introduction to Quantum Mechanics
Quantum mechanics is a set of physical theories that emerged in the twentieth century to explain phenomena at the level of atom and subatomic particles and have been combined between particle properties and wave properties to show the expression of the double-wave duality. Quantum mechanics is thus responsible for physical interpretation at the atomic level and also applies On classical mechanics but does not show its impact at this level, so quantum mechanics is a generalization of classical physics for the possibility of application at the atomic and normal levels.
The name Quantum mechanics refers to the importance of quantum in its construction (a physical term used to describe the smallest amount of energy that can be exchanged between particles and is used to refer to specific energy quantities emitted intermittently, not continuously). The terms quantum physics and quantum theory are often used as synonyms for quantum mechanics. Some writers use the term quantum mechanics to refer to non-relative quantum mechanics.
General introduction
Quantum theory came in the early 20th century such as the theory of relativity to solve problems that classical physics could not explain. Some of these problems can be summarized as follows:
- Contradictions in the classical physics conception of the shape of the atom at the time: in the early twentieth century was conceived of the shape of the atom is similar to the shape of our solar system, where the nucleus is centered in the center and revolves electrons around. However, according to the principles of classical physics itself, the electrons in this model will be subjected to acceleration of central attraction as a result of its rotation around the nucleus, which will lead to electromagnetic radiation, which in turn result in the electrons will lose its energy gradually and consequently approaching the nucleus to collide in a fraction of a second. So the need for a new theory gives another example of maize formation.
- Classical theory also considers that the atomic spectrum must cover all wavelengths with the same intensity, but physicists have noted that experimental results strongly contradict this, as different atoms produce light waves with very specific wavelengths.
-
Another problem arises when we consider the problem of the black body, "an object that absorbs all the radiation falling on it to be completely re-released." All attempts based on traditional statistical physics failed to explain the blackbody radiation curve, especially at high frequencies, Scientists have shown that the laws of thermodynamics have become unable to explain this phenomenon.
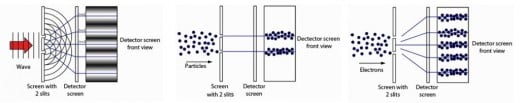
Quantum theory by waveform visualization
Quantum formulations do not provide accurate measurements of the properties of measured particles but give predictions of any possible distributions of all the values that a specific property of the particle can take. The quantum state of the particle includes possibilities for its measurable properties such as position, amount of motion, energy and angular momentum, Quantum mechanics does not give you the precise position of a particle but only gives the probability of its existence at any point in the studied space where it determines paths in which the presence of the particle is large (that is, its probability is greater than that of the particle) E) But it does not eliminate the possibility of being at any point in the vacuum and you can say the same thing about all other characteristics.
However, certain cases remain, including precise values for certain characteristics. These cases are called special cases.
Schrodinger's equation describes the evolution of the wave function with time and thus accurately predicts the quantum states of the particle at any given moment. This gives us a fixed law explaining the evolution of wave functions with precision. These functions have all the position values and the potential amount of motion. The movement predicts that the center of the beam will move with time at constant velocity. At the same time, the extension of the wave will increase to the position more and more indefinitely. There are also some stable quantum systems that do not show a change over time such as the state of the electron in the hydrogen atom Stable circular facial possibility is the presence of a large electron within a certain distance from the nucleus at least while the potential gradually as we move away from the nucleus. The Schrödinger equation introduces an inevitable evolution of the wave function (this evolution is called U). It precisely defines the function values in all vacuum points at any given moment, but the potential nature of quantum mechanics arises from interference in the measurement process to determine one of the measured properties of the particle. The measured property takes any of the available values according to their probability value.
NEXT article will be dealing with Quantum Mechanics explanations.
© 2019 Oussema Ben Romdhane