Study of some components in Urban Soil Samples using the Gas Chromatograph/Mass Spectrometer (GC/MS).
Introduction:
Anthropogenic substances have continually entered into natural cycles for as long as they have been synthesized. The will exist and will continue to exist in those area with high population density, and also, in areas sparsely populated due to agents of erosion and nature. The soil sample was collected from a residential neighborhood in East Brooklyn 2348, East 13th Street, Brooklyn, New York. The sample was initially sifted to remove any excess plant material and heated to dry the soil and to drive off any volatile organic compounds. The addition of methylene chloride signified the initial process of separating the soil particles with the sonicator agitating the particles in the sample via ultrasound energy by breaking intermolecular interactions.
For the florisil cleanup, cotton and sand was placed first in the chromatography column to prevent the florisil from clogging the holes. The florisil clean up of the concentrated sample was done in two parts, first with a 15% ethyl ether and petroleum ether solution and then again with a 50% ethyl ether/ petroleum ether solution. The 50% ethyl ether/petroleum ether clean up, being more polar than the 15% solution, will draw out the more polar compounds present in the sample.
Both the 15% and 50% eluants were then analyzed via the GC/MS and correlated with the National Library of Medicine to obtain environmental information on the findings.
Experimental:
(i) Sample preparation: Approximately 30-35 grams of soil sample was freed of debris and mixed with approximately 10 grams of anhydrous sodium sulfate. The sample was then left overnight in a drying over at 150°C.
(ii) Extraction: Approximately 30 grams of the dried soil sample was mixed with 30mls of methylene chloride. The soil/methylene chloride mixture was then placed into the sonicator for approximately 3-4 minutes and then vacuum filtered. This process was repeated once more using the residue from the filtration. The combined eluate was then transferred to a 100ml round bottom flask and placed in the rota-vapor until a thin film formed. The round bottom flask was washed with approximately 2-4mls of methylene chloride, with the concentrate transferred to a sample vial.
(iii) Florisil Clean-up: A chromatography column was set up on a ring stand, with a small amount of cotton, followed by approximately 2 grams of sand placed to the bottom of the chromatography column. The column was half filled with florisil and then petroleum ether. The petroleum ether was allowed to fully saturate the florisil and then drained through until it was approximately half of a centimeter above the florisil. The soil eluate, collected in the sample vial from the rota-vapor, was then poured into the chromatography column and the sample vial was further rinsed with petroleum ether, and poured into the chromatography column. 100 mls of 15% ethyl ether/petroleum ether was measured and poured slowly into the chromatography column and allowed to drain through, with the 15% ethyl ether/petroleum ether – soil eluate collected in a 125 ml Erlenmeyer flask. 50% ethyl ether/petroleum ether was then poured into the chromatography column, and allowed to run through. This was collected in another Erlenmeyer flask and placed aside. The 15% ethyl ether/petroleum ether- soil eluate was then transferred to a round bottom flask (rinsed with acetone and 15% ethyl ether/petroleum ether) and then placed into the rota-vapor. The round bottom flask was then rinsed with about 5mls of 15% ethyl ether/petroleum ether and collected in a sample vial. This was then repeated with the 50% ethyl ether/petroleum ether- soil eluate sample, using 50% ethyl ether/petroleum ether to rinse the round bottom flask instead.
(iv) GC/MS: both samples were then analyzed with the GC/MS. See attached chromatograms.
Identification of Components:
The GC/MS attempted to identify the components in the soil sample through correlation with an internal library.
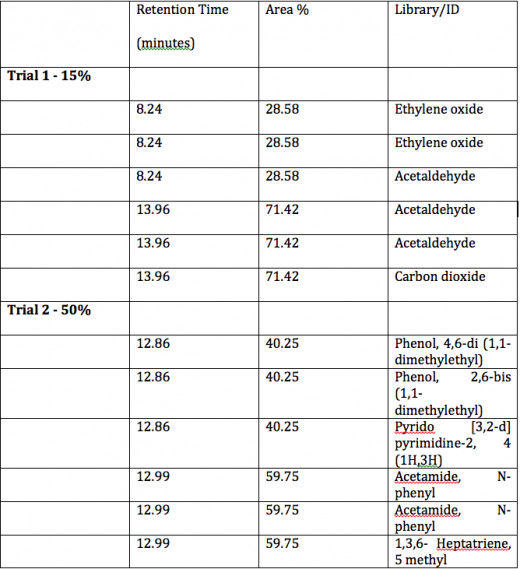
As per the attached GC/MS reports, the sample was found to contain 83% of Acetamide, n-phenyl, commonly known as acetanilide.
Environmental Information on Soil Components
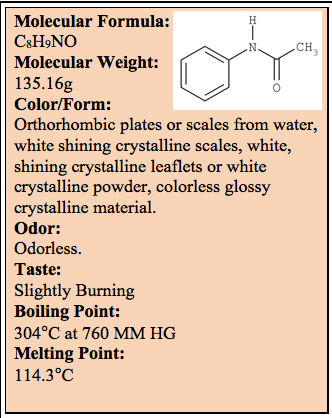
According to the Hazardous Substances Data Bank, Acetanilide may be released into the environment via various waste streams from production. It is used as an intermediate in drug and dye production, as a stabilizer in hydrogen peroxide and cellulose esters, as a plasticizer and as a rubber accelerator. Based on Henry’s Law constant of 6.2 x 10-9 atm-cu m/mol and an extrapolated vapor pressure of 1.2 x 10-3mm Hg, acetanilide is not expected to volatilize from either wet or dry soil. Hydrolysis, adsorption to soil and sediment and volatilization are not expected to be environmentally important removal processes in aquatic systems.
One limitation of this experiment was the soil sampling method. To ensure an accurate analysis of the results, several samples should have been collected from various points within the sampling area. A much more precise inference could be made, as the presence of acetanilide can be attributed to the use of herbicides and pesticides, if it could be proven that acetanilide was indeed a common factor in various samples from the area. According to the U.S. Department of Health and Human Services- Household Products Database, the herbicide Alachlor contains roughly 25-40% acetanilide. The EPA states that alachlor is the second most widely used herbicide in the United States. In soil, it is transformed to its metabolites, primarily by biodegradation, with the half life of alachlor being approximately 15 days. The location of the sample site, Sheepsheads Bay, Brooklyn tends to have soil of sandy texture, hence the presence of continuous pores or channels within the soil will increase the mobility of the alachlor in the soil, possibly, seeping into groundwater sources.
Another possibility is the use of acetanilide as a rubber accelerator, where there might have been remnants of a tire or rubber hose within close proximity to the sample site.
References
Technical Factsheet on Alachlor. U.S. Environmental Protection Agency. Ground Water & Drinking water. <http://www.epa.gov/OGWDW/dwh/t-soc/alachlor.html>
Household Products Database. U.S. Department of Health & Human Services. Health & Safety information on Household Products- Alachlor. <http://hpd.nlm.nih.gov/cgi-bin/household/brands?tbl=chem&id=3132&query=acetanilide&searchas=TblChemicals>
ToxnetÒ United States National Library of Medicine. National Institutes of Health. <http://sis.nlm.nih.gov/enviro.html>