The Different Types Of Chromatography

What is chromatography?
Chromatography is the process of separating substances by their movement through or over a separating material. The name 'chromatography' comes from the Greek word 'chroma' meaning colour and 'graphein' meaning to write.
The basics of chromatography
During chromatography a mobile phase will sweep a mixture over the stationary phase. The basic principle of chromatography works on the basis that different components have different affinities for a stationary phase and a mobile phase. This hub will go over two different types of chromatography - thin layer chromatography (TLC) and gas chromatography (GC). In TLC the stationary phase is a solid and mobile phase a liquid, in GC the stationary phase is a liquid or a solid support and the mobile phase is a gas.
Efficient separation of compounds depends on two main factors; adsorption or relative solubility.
- A solid stationary phase will separate by adsorption, as the mobile phase passes over the stationary phase the components are able to bind to the solid's surface.
- A liquid stationary phase separates by relative solubility as some components will be more soluble in water and are thus slowed down more than components will lower solubility.
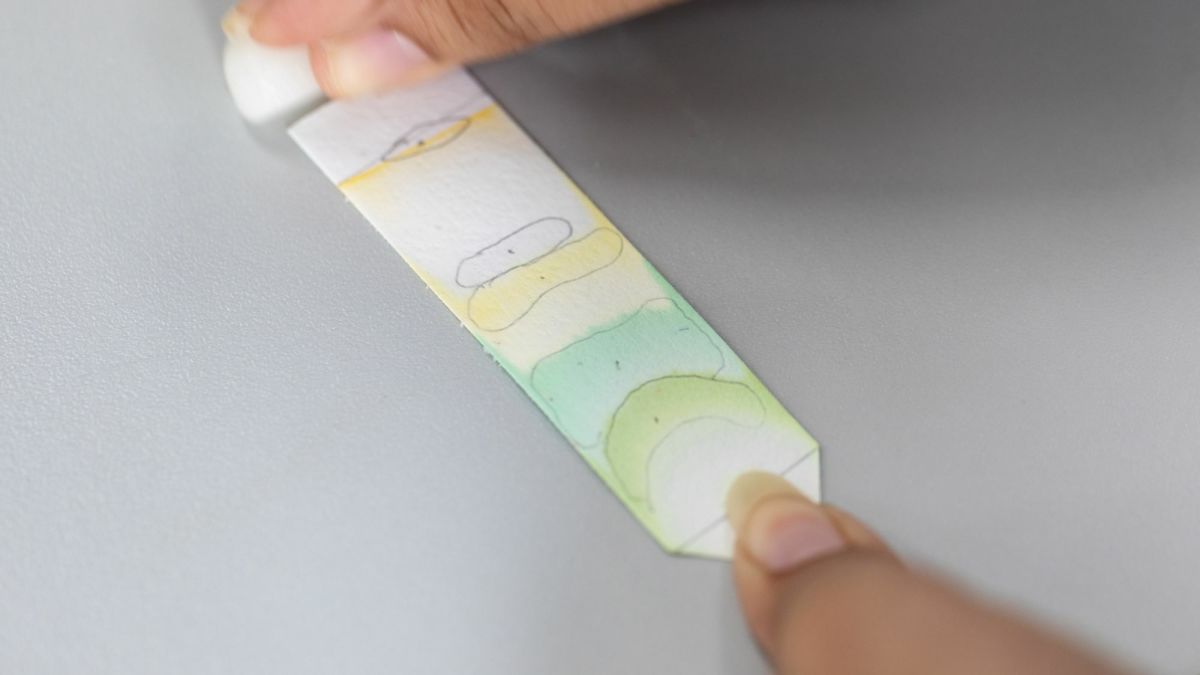
Make your own chromatogram!
What you will need:
- Filter paper.
- Ink pen.
- Smallish container (an empty yoghurt pot would work nicely).
What to do:
- Cut a strip out of the filter paper so that it is long enough that you can put it in the container and it will rest over the side.
- Colour a large spot towards one end of the paper (leave a bit of room from the end) with a coloured felt pen/ink pen.
- Fill the bottom of the container/pot with a small amount of water but not enough so that when you dip the paper in it will submerge the ink spot.
- Dip the paper 'tail' in the water (the end with the ink spot), and rest the other end of the paper on top of the container/pot.
- Gradually, the water will travel up towards the ink spot and as it goes past will carry the different colours of ink in the ink spot with it.
- Results should start to appear in 5-10mins.
- Remove the paper and allow to dry, you now have your own chromatogram!
Thin-layer chromatography (TLC)
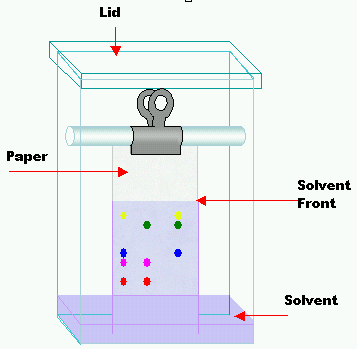
TLC is a quick and simple analytical method which is often used to check the purity of compounds. The stationary phase is a thin layer of an adsorbent such as silica gel or alumina coated on a flat inert support such as a sheet or glass or plastic which is known as a thin layer chromatography plate. The mobile phase is a liquid solvent which moves vertically up the TLC plate.
The chromatogram is produced by the following steps:
- A small sample of the mixture is dissolved.
- A small spot of the sample mixture is placed on the TLC plate at one end and a pencil line is drawn where the spot is.
- The sample spot is allowed to dry and the TLC plate is placed in a jar containing a shallow layer of solvent.
- The solvent must be below the sample spot to ensure that the solution won't wash the sample off of the TLC plate.
- The jar is sealed so that non of the solvent vapour escapes and the inside of the jar is saturated with the solvent vapour.
- As the solvent rises up the TLC plate then it meets the samples and the different components in the mixture rise up the plate with the solvent.
- The compounds then bind to the stationary phase surface by adsorption - some components bind more strong than others which results in the different components being separated at different differences up the TLC plate.
- Maximum separation is achieved by letting the solvent rise fully up the TLC plate. It is then taken out of the solvent and the position of the solvent front is marked on the plate with a pencil line and then allowed to evaporate - this results in a chromatogram.
- Each component in the original solution shows up as a spot on the plate and if they are colourless then a locating agent/radiation may be used.
Rf values can be used to show how far a component has moved compared to the solvent front.
- Rf = the distance moved by the component / distance moved by solvent front.
Rf values of components can be compared with those of known pure compounds to identify unknown compounds. You can also put known substances in the starting solution to make it easier to identify a component by comparing distances travelled by the unknown component and the known components.
Gas chromatography
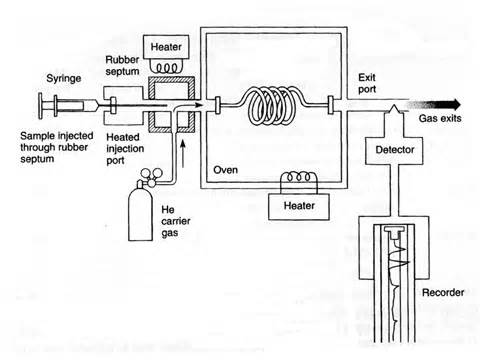
Gas chromatography is normally used to separate volatile components. It takes place in an gas chromatogram.
- The stationary phase is a thin layer of a solid or a liquid coated on the inside of a tube which acts as the solid support.
- The tube is wound in a coil and fits inside a thermostatically controlled oven.
- The liquid for the stationary phase is often a long chained alkane with a high boiling point or a silicone polymer.
- The mobile phase is an unreactive carrier gas such as helium or nitrogen.
The chromatogram for gas chromatography is made using the following steps:
- The mixture is injected into a gas chromatograph where it is vaporised and the mobile gas then pushes the mixture through the column and the components slow down as they dissolve in the liquid stationary phase or adsorb to the solid stationary phase.
- The components with stronger adsorption or greater solubility will be slowed down more than the other components and thus they separate.
- Each component will leave the column at a different time and a computer processes the results and produces a gas chromatogram.
- The time taken for the component to pass through the chromatograph is called the retention time.
- Retention times can be compared with retention times of known compounds to identify the compounds.
Limitations
Limitations of TLC -
- Similar compounds have similar Rf values.
- If solvents are very soluble then they may be washed up the plate in the solvent front.
- It may be difficult to find a suitable solvent that separates all of the components.
- Unknown compounds will not have Rf values to compare to.
Limitations of GC -
- GC may not accurately identify all compounds as compounds may have the same retention times or peak shape on the chromatogram.
- A small amount of substance can go unnoticed if it has the same retention time of another substance with a higher concentration.
- Much like TLC unknown compounds have no retention times to compare to.