The Science behind Food

Confession: I have a huge crush on Food Network’s Alton Brown, I always go for the nerd and he brings together the wonderful world of science with the wide world of food. I respond well to his approach, as predominantly seen on his show Good Eats where he explains, in detail, the process behind cooking which most times leads to science. The science behind food is not only interesting to a geek like me but it is useful; nutritionists, dieticians, and fitness experts really understand what is in our foods and what are body needs to achieve ultimate health and fitness. The science behind cooking needs a foundation; it needs the science behind food first. And that’s where we’ll start.
Three of the four major building blocks of the body are ultimately the same major components in our diets- carbohydrates (sugars), lipids (fats), and proteins. The body’s fourth building block is nucleic acid, DNA and RNA. Thinking back to my time in school, nucleic acid digestion is not something I remember. But when brainstorming this article it was clear to me our foods contain their own DNA and we eat food, so what happens to outside sources of DNA in our bodies? Our bodies digest it. However it is not a nutrient since our bodies can make it without ingesting components for it. DNA from food is an interesting side topic but for the purposes of this discussion we'll leave it out.
Monosaccharide Components
| =Disaccharide
|
|---|---|
Glucose + Glucose
| = Maltose
|
Glucose + Fructose
| = Sucrose
|
Galactose + Glucose
| = Lactose
|
Carbohydrates have different names representing the composition and complexities of the molecules. The simplest sugars are glucose, fructose, and galactose. Combinations of multiple simple sugars, either units of one of the above or combinations of two above give us lactose, sucrose, and maltose. The simplest sugars are monosaccharides; the next step up, lactose for example, is disaccharides where the monomers are held together by glycosidic bonds. Polysaccharides are made up of anywhere from a hundred to a few thousand monosaccharides. In humans when sugars from food aren’t immediately used they are stored as glycogen in the liver and muscle cells. Speaking of storage forms of carbohydrates- the foods we eat also have storage forms of glucose. In the case of animal food sources, glycogen is generally not a contributor to our diet since at the time of slaughter it is quickly broken down. Starch, the plant storage form of glucose, does contribute significantly to our diet. Our body breaks it down to its simplest component glucose for various bodily uses. Fiber, an often talked about dietary component, is generally known as non-starch polysaccharides although there are some non-polysaccharide fibers in addition. The human body does not contain enzymes to break down the bonds that hold monosaccharides together in fiber. Thus these monosaccharides are not absorbed and cannot be put to use by the body.
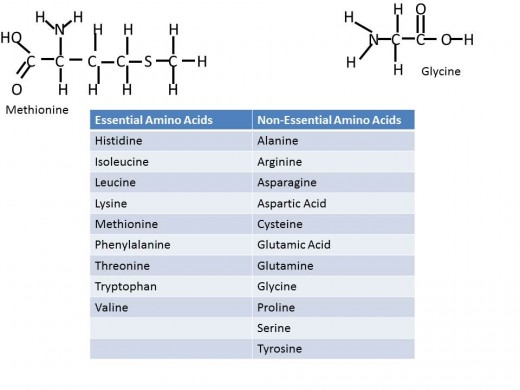
Proteins are also made up from monomers, where its monomer is an amino acid. Amino acids are strung along to make peptides which are held together with peptide bonds. There are 20 common amino acids that make up proteins, each amino acid has a common structure, a carbon bound to an Amino group (NH2), a Carboxyl group (COOH) and a hydrogen. The R group is the final component bonded to the carbon and it is different for each of the twenty amino acids. These twenty are split into two categories in the context of nutrition- those that are essential and those that are non-essential. In nutrition, essential indicates that a person must obtain this component from their diet while non-essential tells the body can build it even if absent from one's diet. Of the 20 common amino acids- nine are essential, including methionine and tryptophan, and eleven are non-essential, including glycine and proline. Proteins have so many diverse functions in the body providing support, storage, and transport. As well enzymes are proteins and collectively enzymes play a role in most every aspect of the human body function.
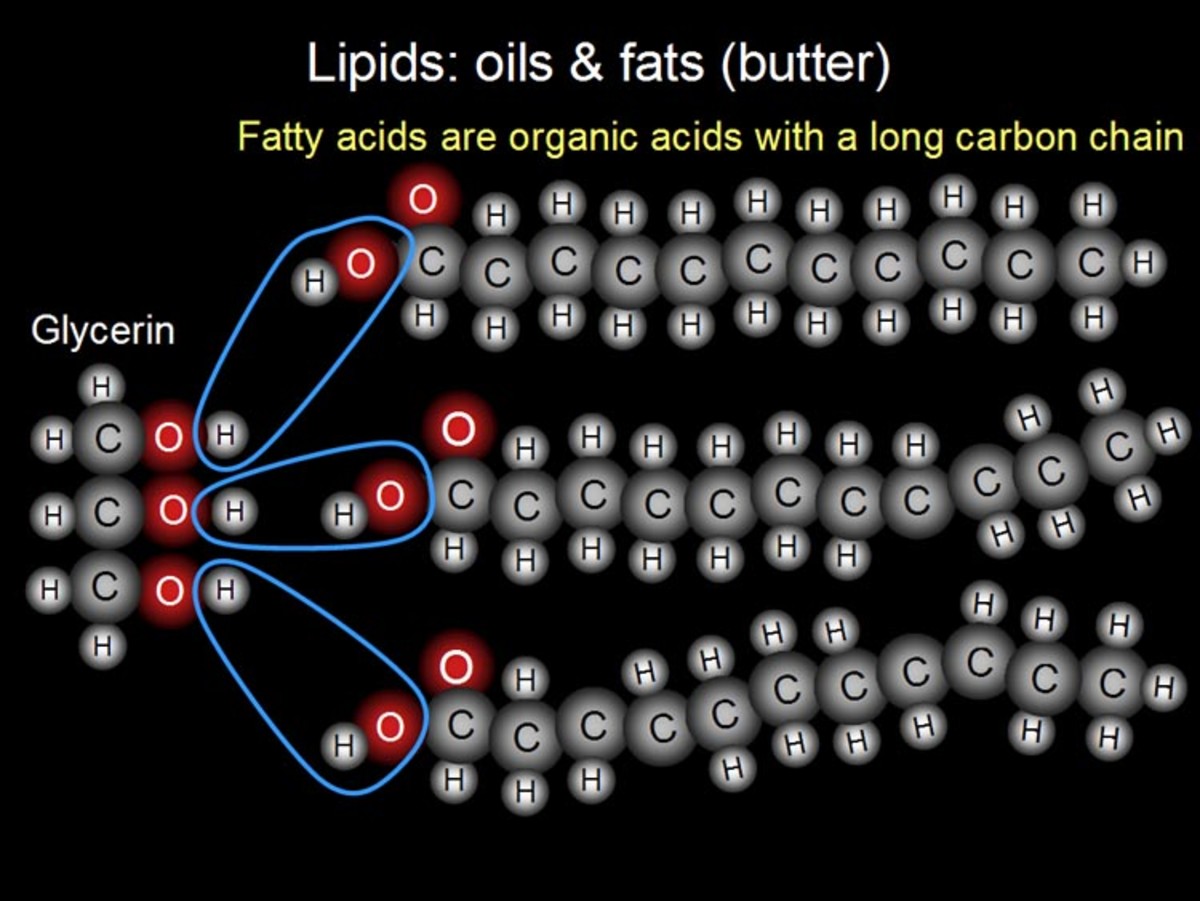
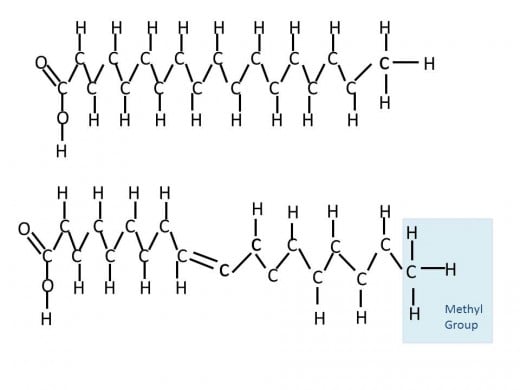
The third major nutrient is lipids; they are constructed a little different than carbohydrates and proteins. Instead of a uniform monomer, monosaccharide or amino acid in the case of the others, the simplest form of a lipid is constructed from two separate components- glycerol and fatty acids. Specifically a triglyceride, or triacylglycerol, is made up of one glycerol and three fatty acids. Glycerol is an alcohol with three carbons while a fatty acid is a long carbon chain of typically 16 to 18 carbons. The three fatty acids can all be the same or different ones, where the difference lies in length (carbon number) and location of double bonds. The presence or absence of double bonds on carbons in the chain give the names we hear often in conversation about what is in our foods. Fats are commonly referred to as saturated or unsaturated. These terms confuse me a bit, but the easiest way to think about it is the long carbon chains are completely saturated with hydrogens in the case of saturated fats. The alternative is unsaturated which by comparison is lacking in hydrogen atoms compared to fully saturated fats.
In order for carbon to be stable in a given molecule it needs to have its outer four electrons bonded to electrons from other sources. In a given long carbon fatty acid, if we take away a hydrogen atom, the carbon needs to compensate for this by making an additional bond between its forth, now unpaired, electron and another electron in a nearby atom. The easiest way to do this it to make a double bond with an atom it is already bonded to and thus we get carbon double bonded to the adjacent carbon in the chain. This means there is always a pair of hydrogens missing from an unsaturated fatty acid, since two neighboring carbon atoms would both need to be missing a hydrogen in order to double bond with each other. There can be more than one double bond present in a given fatty acid and thus more pairs of 'missing' hydrogens. When these double bonds are present they spatially give the long carbon chain kinks. Think about an elevator full of people at an office, it is the last day of one person in the elevator and they are carrying a box of things from their desk. Now not as many people can pack tightly into the elevator because of this one person. Likewise in a food where unsaturated fats are present the molecules cannot tightly pack in and form a solid at room temperature, instead the food remain liquid. On the other hand, saturated fats can tightly pack in and thus can form solids at room temperature. Another term often used with fats is hydrogenated, in which a naturally unsaturated food is made to be saturated- this is done to prevent the lipids from separating into liquid,for example in peanut butter. Nowadays a trip to the peanut butter aisle gives many options including natural peanut butter that contains no hydrogenation and thus can separate out overtime within the jar.
The Vitamins
Water-Soluble
| Fat-Soluble
|
|---|---|
Vitamin C
| Vitamin A
|
B6
| Vitamin D
|
B12
| Vitamin E
|
Thiamin
| Vitamin K
|
Riboflavin
| |
Niacin
| |
Folate
| |
Biotin
| |
Pantothenic Acid
|
Other than Vitamin C, the other eight water soluble vitamins are all Vitamin B variations.
Vitamins, minerals, and water are the other major nutrients in our diets. The major distinction between these three and the three already discussed is these are not energy-yielding nutrients. Carbs, lipids, and proteins- when broken down during digestion- provide energy for the body while vitamins, minerals, and water do not. Instead, they provide structural contributions or play a role in key reactions in the body to name a few examples. Minerals are the simplest nutrient; they are elements direct from the periodic table. In terms of cooking and digestion in the body this means they are durable-high temperatures won’t break them down and we absorb them as is. Minerals are used to build up bone and teeth, as well as necessary co-factors in many enzymatic reactions in the body. Water is a step up in classification from the minerals, being composed of two minerals. Water is the backdrop for almost every major thing going on in the body and we are chiefly composed of it so it’s no wonder it is an essential nutrient.
Vitamins are a bit more fragile in comparison to minerals and water, they are organic compounds (carbon-containing) like the big three discussed already. As mentioned the latter three nutrients do not provide the body with energy, however vitamins play a key role in energy release in carbs, lipids, and proteins. It is important to know they are a bit delicate because it means certain storage and cooking methods can damage them and render them useless when the body takes them in. They need to remain in their original form to be any good to us. Heat, light, and chemical damage can all take place, as well over-washing foods containing vitamins can wash them right away. One must take care when handling vitamin-rich foods and not assume they are getting to the body intact if they were improperly stored or cooked.
The complex and interesting process of digestion in the human body will be briefly summarized here. For certain foods, enzymes are present as early as the mouth to start breaking bonds. Along the alimentary canal, the digestive pathway, other enzymes break down the big three into various sub-components. For each separate class, several enzymes are located along the digestive tract to pick up where the last enzyme left off. For example, proteins are broken down into large polypeptides, then smaller polypeptides, then peptides, and ultimately single amino acids where each step is accomplished by different enzymes along the way. All of this leads up to the smallest components possible for carbs, proteins, and lipids- monosaccharides, amino acids, and glycerol and fatty acids- in the small intestine where absorption into the bloodstream and lymphatic system occur. From there the components will be carried by blood (amino acids and monosaccharides) or lymph (fats which immediately reconstitute after absorption) through their respective circulatory systems to various sites throughout the body.
Now that we have looked at the basic building blocks of nutrition we can get to the heart of the matter in the next installment- the science behind cooking. In this accompanying article, we'll look at a specific dish with multiple ingredients and see how cooking happens on a scientific level.
Part Two
- The Science behind Cooking- Part Two
The second part of the series looking at the science involved in cooking. Here a look at what happens, scientifically speaking, when chicken broccoli ziti is made.