Valence Bond Theory
Explaining Covalent Bonding
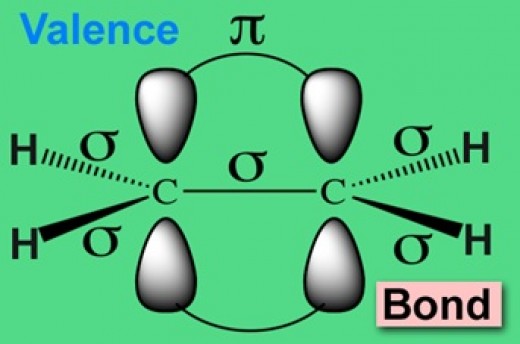
Valence bond theory accounts for bonding between two atoms in terms of overlapping atomic orbitals.
For example, the bonding between two hydrogen atoms is said to be due to the overlap of the 1s orbitals of the two atoms. In polyatomic molecules, the concept of hybridization is invoked in order to make the idea of overlapping orbitals consistent with the molecular geometry.
Explaining Bond Angles
In general, we can correlate the steric number to the hybridization of the atom.
- If the steric number is 2 (linear center), the hybridization is sp.
- If the steric number is 3 (trigonal planar center), the hybridization is sp2.
- If the steric number is 4 (tetrahedral center), the hybridization is sp3.
- If the steric number is 5 (trigonal planar center), the hybridization is dsp3 (or sp3d).
- If the steric number is 6 (octahedral center), the hybridization is d2sp3 (or sp3d2).
Watch those nice videos:
Exercise: What type of orbital hybridization explains the linear electronic geometry around an atom with a steric number of 2?
A. sp
B. sp2,
C. sp3
D. sp3d
E. sp3d2
Answer:
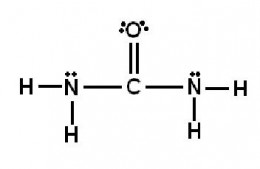
Exercise:What is the hybridization of the valence orbitals of the carbon atom in the structure?
A. sp
B. sp2
C. sp3
D. sp3d
Answer:
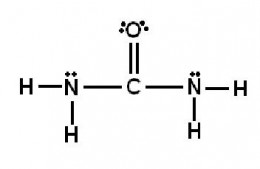
Exercise: In the structure below, the sigma bond between which two atoms is due to an sp2-to-sp2overlap?
A. C and O
B. C and N
C. N and H
Answer:
Explaining Bond Lengths
Hybridization can also be used to explain the effect of multiple bonding on bond lengths.
For example: the C-to-C bond in H3C-CH3 is longer than that in H2C=CH2. The C-to-C bond in the former involves an sp3-to-sp3 overlap, while the latter involves an sp2-to-sp2 overlap. For a hybrid orbital with a greater "p character," the region of high electron density stretches out farther from the nucleus; sp3 hybrids have higher p character than sp2 hybrids.
Exercise and Answer in below video:
References
© 2015 Discover the World