Pembrolizumab, Keytruda® New Cancer Treatment
What Is Pembrolizumab?
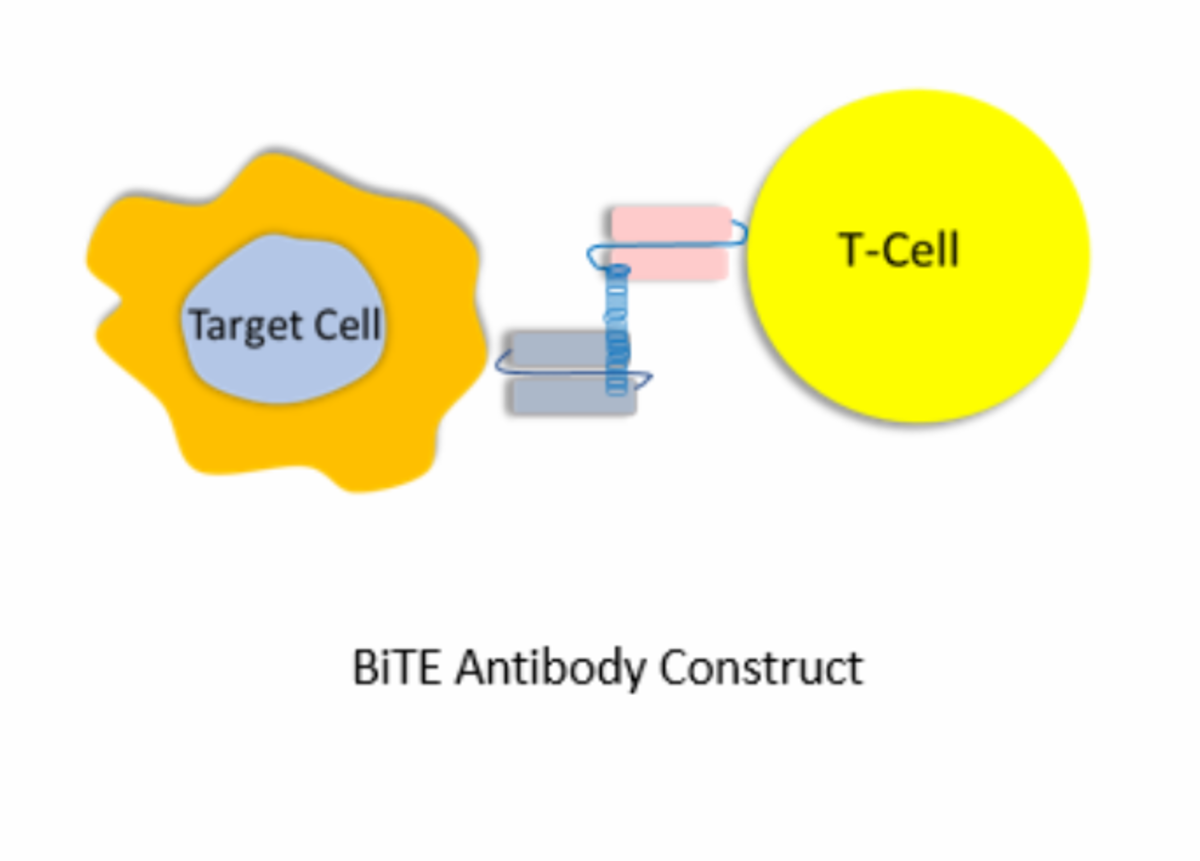
It is a humanized monoclonal immunoglobulin. It means that it is a clone of a single human antibody. Its function is to produce the white blood cells called lymphocytes T, destroy or kill other malignant cells. It is an antineoplastic drug (medicine to treat cancer), which manages certain types of human cancers, which cannot be eliminated by surgery or other therapeutic options. It works by activating the human body's immune response.
In a nutshell, This medicine activates the human body's immunological system's natural response fighting the cancer cells and destroying them.
How It Works
It is an immune therapy. It produces a response from the body's self-defense mechanisms or immune system to fight the malignant cells or cancer cells by activating the appropriate immune response.
Pembrolizumab occupies or blocks a receptor located on the cancer cell's surface called the programmed death -1 (PD-1) receptor. After the PD-1 receptor is blocked, the white cells, lymphocytes T, are activated. Those activated lymphocytes T generate the cellular immune response against the tumor cells, killing or destroying the cancer cells.
When functioning correctly, T cells are triggered by the presence of tumor cells or cancer cells. Therefore when one cancer cell is detected, they attacked and killed it. Cancer cells secrete a chemical product, which occupies the PD-1 receptors and inhibits the natural body immune response.
Cancer cells can be deceiving. Consequently, cancer cells inhibit or prevent the human natural white cells' functions. Its action mechanism is to recover the healthy lymphocytes' T functions and protective capacities against tumor cells.

Pembrolizumab Indications
It is indicated for the treatment of certain types of cancer, which surgical procedures cannot eliminate to remove the affected organ or other anti-cancer therapies, or in those diseases that have spread around the body, which is called metastasizing. The last tag indication was granted on September 22, 2017, to treat gastric and esophageal junction cancer. It is currently approved as the only treatment or in combination with other anti-cancer drugs for the following types of cancer
· unresectable or metastatic melanoma
· metastatic non–small cell lung cancer
· nonsquamous non–small cell lung cancer
· recurrent or metastatic head and neck squamous cell carcinoma
· adult and pediatric patients with refractory classical Hodgkin lymphoma (CHL),
· Locally advanced or metastatic urothelial carcinoma
· Colorectal cancer
· Metastatic gastric or gastroesophageal junction cancer
The list is growing thanks to the FDA's accelerated approval regulations, which consider the tumor response rate and duration of response or time without cancer among patients on therapeutic clinical trials to speed up its approval.
How Patients Receive It
It is administered as an intravenous solution, which means that the nurse inserts a small needle or cannula into the patient's vein in the arm or chest and connects it to a drip, which will administer the drug over 30 minutes, every three weeks.
How Long Does a Patient Need to Stay on Pembrolizumab Treatment?
There is not a precise time frame for the treatment duration. It depends on several individual factors related to:
- The cancer response, the treatment should be administered as a single dose every three weeks as long as the cancer cells' activity is reducing, and some remission (improvement) of the disease is observable. the average duration of the treatment is two years.
- The patient immunological status, Pembrolizumab, induces the destruction of the malignant cells or cancer cells by the lymphocytes T, which form part of the normal human body immune response. However, either the disease or the drug might affect the immune response, preventing the drug's appropriate action mechanism and altering the healthy body cells.
- The presence of any intolerable side effect; treatment must stop if any severe side effect becomes sharp enough, or the drug shows any toxicity sign.
- Patients will receive the treatment with Pembrolizumab based on their experience with the drug; for as long as the treatment works, reducing the malignant cells' size or the cancer body's harmful effects. The average treatment duration is 24 months or 8 Pembrolizumab's doses.

What are the Most Common Side Effects?
- Immune-mediated induced colitis is an inflammation of the bowel, which produces severe diarrhea and malabsorption of nutrients from food or pills.
- Immune-mediated induced pneumonitis is the inflammation of the lung tissue, which is not produced by an infection. The lung tissue inflammation might block the respiratory process and produce air grasping. In severe episodes, it may be a life-threatening condition.
- Immune-mediated hepatitis is the inflammation of the liver, which is neither caused by infection nor by toxics.
- Hypophysitis is the inflammation of the pituitary gland, and it produces severe body imbalances due to wrong regulations of various essential human body hormones. The worst presentation affects the adrenal glands.
- Type I diabetes mellitus is the deficiency in insulin hormone production, which increases blood sugar levels.
How Doctors Check for Side Effects and Disease Progression?
- Doctors will require patients to inform and report any of the critical signs or symptoms to watch for side effects or toxicity.
- Several X-rays and MRIs may be done to assess the disease stage.
- Performing lab´s tests is part of the standard of care.
References
1. First Immunotherapy Approved for Gastric Cancer. https://www.medscape.com/viewarticle/886114
2. FDA Places Clinical Hold on Merck's Combo Therapy for Multiple Myeloma https://www.medscape.com/viewarticle/882518
3. pembrolizumab (Rx) https://reference.medscape.com/drug/keytruda-pembrolizumab-999962
4. Cancer Drugs Dominate New FDA Watch List https://www.medscape.com/viewarticle/886843
This content is for informational purposes only and does not substitute for formal and individualized diagnosis, prognosis, treatment, prescription, and/or dietary advice from a licensed medical professional. Do not stop or alter your current course of treatment. If pregnant or nursing, consult with a qualified provider on an individual basis. Seek immediate help if you are experiencing a medical emergency.
© 2017 Dr Aron Mejias