What are Alkanes?
Alkane Facts and Things You Should Know!
Learning about alkanes is easier than you think. With these short and simple points, you will learn the basics and further down the article you learn how to draw them. Unless you want to know what they are used for! Even then, the explanation is relatively simple and easy to understand. So what do I absolutely need to know?
- Alkanes have single bonds between the carbon and hydrogen atoms, which we call covalent bonds.
- Alkanes are always saturated as they have no spare bonds.
- Alkanes do not decolourise bromine water. Putting a hydrocarbon into bromine water tests for alkanes and alkenes. If the bromine water stays the same colour it is an alkane, if it turns transparent or colourless then it is an alkene.
- Alkanes do not form polymers.
- Alkanes burn cleanly (complete combustion), which means that it only produces two products which are water (H2O) and carbon dioxide (CO2).
- The general formula for alkanes is CnH(2n+2). This formula is explained in detail below.
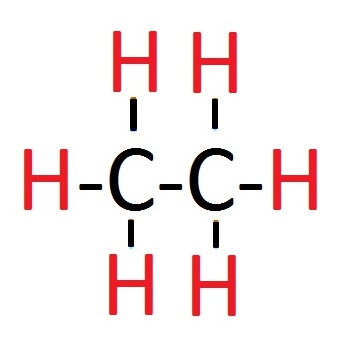
Alkanes General Formula
The general formula for alkanes is CnH(2n+2). To start with, the "C" stands for carbon and the "H" stands for hydrogen. Simply, you are displaying how many carbon atoms there are and how many hydrogen atoms. Any number is "n" but the "n" remains the same in the equation. The bracket after the "H" means two multiplied by the number, "n", and then add two. I think it is better to explain it with an example so here we go:
Example:
Let's take ethane, its general formula is C2H6. This is done by having two carbon atoms, which means that 2 must be "n". So then that means we can work out how many hydrogen atoms we have. Simply do, two multiplied by two - as it is "n" - and then add another two on. That all equals six, therefore we have six hydrogen atoms!
See how easy the formula is to work out!
Displayed Formula
You may or may not see a question in a test asking you for the displayed formula. If they ask you for this, all they mean is to do a little diagram for them. The picture above (adjacent to the general formula explanation) shows you how to display the formula. In this example, ethane is used which has the formula C2H6. All that means is you draw two C's with a bond between them - represented by a line - and then six hydrogens around that, so that each carbon has three hydrogen atoms bonded to it. In other formula's like propane, there will be three carbon atoms so the end two carbon atoms will have three hydrogen atoms bonded to it - whereas the middle carbon will only have two hydrogen atoms bonded to it.
Have you been learning?
How many hydrogen atoms are there to carbon atoms in Alkanes?
What are Alkanes used for?
Alkanes have many uses, but it is mainly the shorter chain alkanes that are the most useful to us. Methane, ethane, propane and butane are the alkanes used for generating electricity, cooking and heating. Propane and butane are used as liquified gas for barbecues and other gas cookers. Butane is the gas in cigarette lighters and both butane and propane are used as a propellant in aerosol sprays.
Generally, alkanes with 5-8 carbon atoms in them are volatile liquids. This means that they are used as fuels and solvents like petrol (gasoline) and naptha. Alkanes with 9-16 carbon atoms in them, play a vital role in the manufacturing of diesel and aviation fuels. Finally, alkanes with more than 35 carbon atoms are used in road surfacing as they are like tar.
The First Six Alkanes
Name of Alkane
| Molecular Formula
|
|---|---|
Methane
| CH4
|
Ethane
| C2H6
|
Propane
| C3H8
|
Butane
| C4H10
|
Pentane
| C5H12
|
Hexane
| C6H14
|
Note: The molecular formula numbers should be in subscript, for some reason I couldn't get them subscript.
The Displayed Formula Is Quite Different To What It Really Looks Like
The Big Alkane Quiz!
view quiz statistics
Now you have learnt the basics of Alkanes!
With this article, you have now learnt the very basics of alkanes! Have you tried the quiz yet? See what you get, to make sure you have learnt what I have written.
Or you may still have a question about alkanes that is bugging you? If you do, then leave a comment and I will get back to you ASAP!
© 2014 crazyduck