Lucid Concept Of, "Functional Group".
Functional Groups are Integral Parts of Complex Organic Compounds
Functional group can be defined as; "single atom or group of some atoms which when displaces a hydrogen atom of hydrocarbon, a special class of organic compounds is formed. For example chlorine when displaces one hydrogen atom of any alkane, a special class of organic compounds called, "chloroalkanes" is formed.
It should be noted that, functional groups can not exist independently, but they can co-exist with some hydrocarbon groups known as, "alkyl or aryl groups".
This means: Organic Compound = (alkyl or aryl group) + (functional group).
For example, CH3OH (Methanol) = -CH3 (Methyl group) + -OH (Hydroxyl group). Therefore, in order to understand the fundamental concepts of functional group, it is necessary first to get an idea about different types of organic compounds which may contain functional group.
The vast variety of organic compounds is classified into two major categories: (a) Simple organic compounds and (b) Complex organic compounds.
Theory of, "Simple Organic Compounds"
These are the compounds which contain only two types of atoms: Carbon & Hydrogen.
- Such compounds are commonly known as, "Hydrocarbons".
- Their common source is, "Petroleum".
- They are also known as, "Parent Compounds", because a wide variety of useful organic compounds are derived from these compounds.
- Examples of such compounds are: Methane (CH4), Ethane (C2H6), Propane (C3H8), Butane (C4H10), Benzene (C6H6), Napthalene (C10H8) etc.
- Chemical reactions of these compounds are comparatively simple, which involve displacement of their one or more hydrogen atoms with some functional group to yield a new compound-called, "Complex Compound".
- Importance of these compounds: (a) They are used as fuels hence are the main source of energy on our planet;(b) They are important raw material to synthesize a variety of complex organic compounds which are very useful in our day to day life.
Theory of, "Complex Organic Compounds"
These are the compounds which contain two discrete parts in their structure:
(a) A hydrocarbon part known as, "alkyl group" or "aryl group" symbolized with "R". (Refer table-A given below); and
(b) A functional group, symbolized with "G". (Refer table-B give below).
- Their general formula therefore is, "R-G".
- These compounds are also known as, "Organic Compounds Containing Functional Group".
- They occur freely in nature in living organisms, and can also be synthesized in the laboratory.
- Examples of such compounds are: CH3Cl (Chloromethane), CH3OH (Methanol), CH3NH2 (Methanamine), C6H5OH (Phenol), CH3-CO-CH3 (Propanone or acetone) etc.
- The chemical properties (means chemical reactions) of such compounds largely depend upon the functional group they carry.
- Importance of such compounds: They constitute a large variety of very useful compounds in our day to day life. They include: Alcohols, Phenols, Ethers, Drugs, Dyes, Proteins, Carbohydrates (i.e. sugars), Enzymes, Vitamins, Hormones etc.
Table-A
Name of hydrocarbon (its formula)
| Name of corresponding hydrocarbon group (its formula)
|
|---|---|
Methane (CH4)
| Methyl (-CH3)
|
Ethane (C2H6)
| Eethyl (-C2-H5)
|
Propane (C3H8)
| Propyl (-C3H7)
|
Butane (C4H10)
| Butyl (-C4H9)
|
Benzene (C6H6)
| Phenyl (-C6H5)
|
Names and chemical formulas of some common hydrocarbons and alkyl or aryl groups derived from them.
Table-B
Name of functional group
| Its formula
| Example of compound (its I.U.P.A.C. name)
|
|---|---|---|
Hydroxyl
| -OH
| CH3OH (Methanol), C2H5OH (Ethanol).
|
Chloro
| -Cl
| CH3Cl (Chloromethane), C2H5Cl (Chloroethane).
|
Bromo
| -Br
| CH3Br (Bromomethane), C2H5Br (Bromoethane)
|
Iodo
| -I
| CH3I (Iodomethane), C2H5I (Iodoethane)
|
Ether
| -O-
| O-CH3 (Methoxymethane), CH3-O-CH2CH3 (Methoxyethane)
|
Ketone
| -CO-
| CH3-CO-CH3 (Propanone)
|
Aldehyde
| -CHO
| CH3-CHO (Ethanal), C2H5-CHO (Propanal)
|
Carboxyl
| -COOH
| CH3-COOH (Ethanoic acid), CH3CH2-COOH (Propanoic acid)
|
Anhydride
| -CO-O-CO-
| CH3CO-O-COCH3 (Ethanoic anhydride)
|
Acid chloride
| -COCl
| CH3-COCl (Ethanoyl chloride)
|
Ester
| -COO-
| CH3-COO-CH3 (Methyl ethanoate)
|
amide
| -CONH2
| CH3-CONH2 (Ethanamide)
|
Amine
| -NH2
| CH3-NH2 (Methanamine)
|
Nitro
| -NO2
| CH3-NO2 (Nitromethane)
|
Nitrile
| -CN
| CH3-CN (Ethanenitrile)
|
Names of some common functional groups, their chemical formulas and examples of some compounds containing the said functional groups.
Importance of, "Functional Group"
In the language of chemistry, functional group means a single atom or collection of some atoms attached to hydrocarbon part of an organic compound through which the compound functions (means reacts). Thus, Functional Group=Reacting Group!
As discussed above, there are two divisions in the complex organic compound:
(a) Hydrocarbon part and
(b) Functional group.
For example, in methanol (CH3OH), the hydrocarbon part is -CH3; while the functional group part is -OH. As the reactivity of functional group is generally higher than that of hydrocarbon part, the complex compound reacts through its functional group.
The chemical reaction of an organic compound is actually chemical reaction of its functional group. This can be shown by equations of some reactions given below. The functional groups are shown by bold letters, while hydrocarbon parts of compounds are shown by normal letters.
The notable point is, carbon and hydrogen atoms of hydrocarbon parts, do not participate in the reaction at all!
This also explains the importance of functional group.
Tips to Distinguish between Hydrocarbon Part and Functional Group
The two parts (the hydrocarbon part & functional group part) of given organic compound as explained above can be identified based on following tips:
(a) Identify first the longest & continuous chain of carbon & hydrogen atoms to which when one more hydrogen atom is added, it will represent a complete hydrocarbon compound. This must be the hydrocarbon part of the compound and is also called, "alkyl or aryl group". e.g. In case of Butanol (CH3CH2CH2CH2-OH), the longest & continuous chain of carbon & hydrogen is the CH3CH2CH2CH2- part, into which when one more hydrogen atom is added, it will become CH3CH2CH2CH3, which represent a complete hydrocarbon-Butane.
(b) The remaining part of compound, containing at least one heteroatom (means an atom other than carbon & hydrogen) should be a functional group. In the example given above, the "-OH" is the remaining part containing "O" (oxygen) as a hetero atom. It is known as "hydroxyl" functional group.
Examples of Some Chemical Reactions Showing Involvement of Functional Groups
(a) CH3-OH + H-Cl → CH3-Cl + H-OH (i.e. H2O).
(Methanol) (Hydrochloric acid) (Chloromethane) (Water)
(b) CH3CH2-OH + H-Br → CH3CH2-Br + H-OH.
(Ethanol) (Hydrobromic acid) (Bromoethane) (Water)
(c) CH3CH2-Br + H-NH2 (i.e. NH3) → CH3CH2-NH2 + H-Br.
(Bromoethane) (Ammonia) (Ethanamine) (Hydrogen bromide)
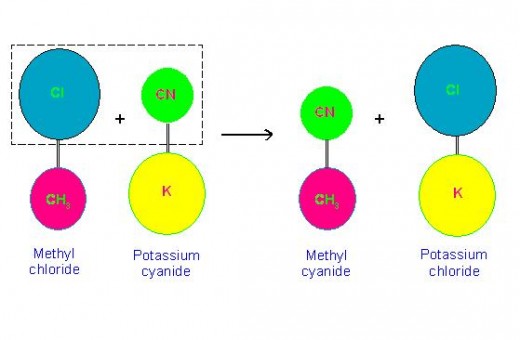
(d) CH3CH2-Br + K-CN → CH3CH2-CN + K-Br.
(Bromoethane) (Potassium cyanide) (Propanenitrile) (Potassium bromide)
These reactions clearly indicate that, it is the functional group of compound which experience a chemical change, and not the hydrocarbon part present in it!
Following picture will make this clearer.
Equations of some Chemical Reactions Showing Involvement of Functional Groups


Benefits of Study of Functional Groups
- Chemical characteristics of complex organic compounds depend upon the functional group they carry. Since functional groups are only few while organic compounds are numerous, by studying chemistry of few functional groups chemical characteristics of a wide range of organic compounds can be studied easily.
- Compounds containing similar functional group resemble in their chemical characteristics. Hence, similarities in the chemical characteristics between any two members of a homologous series (means compounds containing similar functional group arranged in ascending order of their molar mass), can be easily studied.
- The organic compounds are large in number; hence their proper identification requires a systematic approach to assign them a particular name. The "International Union of Pure & Applied Chemistry" (abbreviated as, "I. U. P. A. C.) has issued guidelines for their nomenclature. This I. U. P. A. C. system of nomenclature of organic compounds is based on the functional group of compounds. For example, if a compound contains a hydroxyl group, its I. U. P. A. C. name must be ended with a suffix, "ol". It is due to this reason that names of all alcohols end with "ol"; like: Ethanol, Propanol, Butanol, Hexanol and so on.
- Table-C given below shows prefixes or suffixes used for some common functional groups and names of corresponding compounds.
- By now, we know that the reactivity of organic compound depends upon the functional group it carries. How to compare the chemical reactivity of two different organic compounds containing two different functional groups? For this purpose, relative reactivity order of various functional groups is taken into consideration. Below is the list of some common functional groups which are arranged in the decreasing order of their reactivity. From the study of this reactivity order of functional groups, it is now easy to understand that reactivity of -OH group is more than that of -CHO group. This indicates that alcohols are more reactive than aldehydes, provided they contain same hydrocarbon part. This enables us to identify the more reactive compound from a group of several compounds.
Table-C
Name, symbol of functional group; (suffix or prefix used for it)
| Name of corresponding compound
|
|---|---|
Aldehyde, -CHO; (suffix, "al")
| Ethanal, Propanal
|
Carboxylic acid, -COOH; (suffix, "oic acid")
| Methanoic acid, Ethanoin acid
|
Chloro, -Cl; (prefix, "chloro")
| Chloromethane, Chloropropane
|
Amino, -NH2; (suffix, "amine")
| Methanamine, Ethanamine
|
Ketone, -CO-; (suffix, "one")
| Propanone, Butanone
|
Cyanide, -CN; (suffix, "nitrile")
| Ethanenitrile, Pentanenitrile
|
Ester, -COO-; (suffix, "oate")
| Methyl propanoate, Ethyl ethanoate
|
List of some common functional groups, suffix/prefix used for them & name of compound carrying such functional groups.
Reactivity Order of some Common Functional Groups
-COOH > -COO- > -CN > -CHO > -CO- > -OH > -NH2 .
From this order, reactivity of two different compounds containing different functional groups but same hydrocarbon part can be easily compared.
For example, which one is more reactive, CH3OH or CH3COOH?
Obviously, it is CH3COOH which is more reactive, because the -COOH functional group is more reactive than -OH functional group.
[Note: However, it should be noted that based upon only this much knowledge, relative reactivity of any two organic compounds, can not be compared. e.g. reactivity (means relative strength as a base) of both Methanamine (CH3NH2) and Ethanamine (CH3CH2NH2) are not same as they contain the same functional group. In this case, it is CH3CH2NH2 which is more basic. Likewise relative acidic strengths of some well known acids follow the order: H-COOH > C6H5-COOH > CH3-COOH, which can not be explained easily.
This requires a deeper knowledge of the subject, discussion about which is beyond the scope of this article as it may harm the focus on the topic.]
- Refer the "quiz" and "poll" given below.
Can you justify the Reactivity of Organic Compound Based on its Functional Group?
view quiz statisticsBy now, can you predict Which Compound is More Reactive?
Which one is more reactive, Ethanal or Ethanol?
Study of some Compounds which Contain More than one Functional Group
An organic compound sometimes contains 2, 3, 4 or even more functional groups.
- Examples of compounds containing 2 functional groups:
(a) OH-CH2CH2-OH, known as, "Ethane-1, 2-diol" or "ethylene glycol")
(b) CH2-(NH2)-COOH, known as, "glycine"-an important amino acid.
- Examples of compounds containing 3 functional groups:
(a) OH-CH2-CH-(OH)-CH2-OH, known as, "Propane-1, 2, 3-triol", or "glycerol or glycerine.
(b) CH3-C6H2-(NO2)3, known as, "2, 4, 6-Trinitrotoluene" or "T. N. T." which is a well known explosive.
- Examples of compounds containing 4 functional groups:
(a) OH-C6H2-(NO2)3, known as, "2, 4, 6-Trinitrophenol" or "Picric acid".
(b) C-Cl4, known as, "Tetrachloromethane" or "Carbon tetrachloride".
- Examples of compounds containing 6 functional groups:
(a) C6H6Cl6, known as, "Benzenehexachloride" or "B. H. C.".
(b) CH2OH-CH(OH)-CH(OH)-CH(OH)-CH(OH)-CHO, known as, "Glucose" -a well known sugar.
[Note: (1) The functional group included in parenthesis indicates its relation with carbon atom situated prior to hydrogen atom in the chain. (2) Name of compound written in italic shows its common name and not I. U. P. A. C. name].
The chemical reactivity as well as I. U. P. A. C. nomenclature of above compounds generally depend upon, reactivity of both the functional groups.
How to Take the True Benefit of this article?
The study of chemistry subject requires some basic concepts to digest appropriately. Only repeated reading, writing and cramming can not help much.
For this purpose, one should think over and over after reading it and then try to ask the counter questions to his/her own self. After thorough thinking on the subject, one should derive his/her own personal concepts which are written nowhere. And this is the grip on the subject!
Since chemistry is a vast subject, whatever discussed in this article is not all about functional groups. This is only preliminary information on this topic. If you have passion to understand every thing in a proper way, please do not stop here, go on referring additional literature.
In the course of learning more, the books referred below may help you.
References:
(1) Organic Chemistry, 7th edition, written by, Robert Thornton Morrison & Robert Neilson Boyd
(2) Organic Chemistry, 6th edition, volume-I, written by, I. L. Finar
(3) I. I. T. Chemistry, by Dr. O.P. Agarwal, 135th edition, Jai Prakash Nath Publications, Meerut, India
(4) Pradeep's New Course Chemistry, Class XI, Vol. II, 27th edition, Pradeep Publication, jalandhar, India
(5) Nootan ISC Chemistry, Class XI, by Dr. H. C. Srivastava, Published by: Nageen Prakashan (Pvt.) Ltd., 310, Western Kutchery Road, Meerut-250001, U.P., India
(6) Fundamentals Of Chemistry, Class 11, by J. D. Lee, Solomons & Fryhle, Published by: Wiley India Pvt. Ltd., 4435-35/7, Ansari Road, Daryaganj, New Delhi-110002
(7) Cambridge International AS and A Level Chemistry Coursebook; by Roger Norris, Lawrie Ryan and David Acaster, Cambridge University Press, The Edinburgh Building, Cambridge CB2 8RU, UK
Next
- Live Long! Drink Acid!!
Put, “The End”, to health problems of your child-Give him an acid, now!? Are you health conscious for your child? Here is the information about some such acids, one needs to consume regularly!