Why Do Prescriptions Cost so Much?
Drug Research is Done on Human Volunteers
Why Drugs are Expensive
Do you say ouch every time you pick up a prescription? Have you ever wondered why it takes so long for a new drug to hit the market? When your doctor prescribes a medication, that drug must be approved by the FDA (Federal Drug Administration).
Part of the approval process, though, is the 'clinical trial' stage, where drugs are researched in human subjects (volunteers) to examine safety and effectiveness. And the entire process takes many years - as well as many millions of dollars.
Although some people might think drug companies perform the human research stage of drug production, this is not always true. That part of the research can take many years, and usually required resources beyond the lab-testing done by pharmaceutical companies.
Drug firms (or device firms) generally do development (test tube research and testing in animals) and later produce and market drugs that have been approved.
But the part of research involving human testing is very specialized.
In recent years, Contract Research Organizations (CROs) have become important players in the test-tube-to-market process of creating new drugs.
Contract Research Organizations are firms that are hired by drug companies to research and document drugs or devices before they can be approved by the FDA. All of these steps are there for the safety of those who will eventually take the drugs, and they are all time consuming and costly.
How Drug Research Works (Educational Video by Novartis)
Are Drugs Tested on "Human Lab Rats"?
There are many Contract Research Organizations (CROs) in the United States, and most of them work in similar ways. Although some people might think these firms are using "human lab rats," there are tight regulations for this type of research, and it's essential for drugs to be rigorously tested before they can be prescribed.
Most major firms, such as PPD, Worldwide Clinical Trials and a few others, have resources and facilities to take drugs through all stages of human research. There are three stages of drug research before approval, and another stage for after-market research.
These firms are contracted by drug and device companies to perform objective, third-party testing of drug formulations or investigative devices.
Since the first stage (referred to as 'first in man') is required to be very controlled, larger CROs have in-house units where volunteers can be closely monitored 24 hours a day.
The CRO will recruit appropriate volunteers, thoroughly inform them of what will happen during the research study (a step called the 'Informed Consent' process) and conduct extensive screening tests to make certain they are medically qualified (such as blood and urine tests), as well as other tests to determine if they're suitably qualified.
After the volunteer's lab and other screening tests are evaluated, they will be admitted to the study and, in most cases given a date to return to the facility.
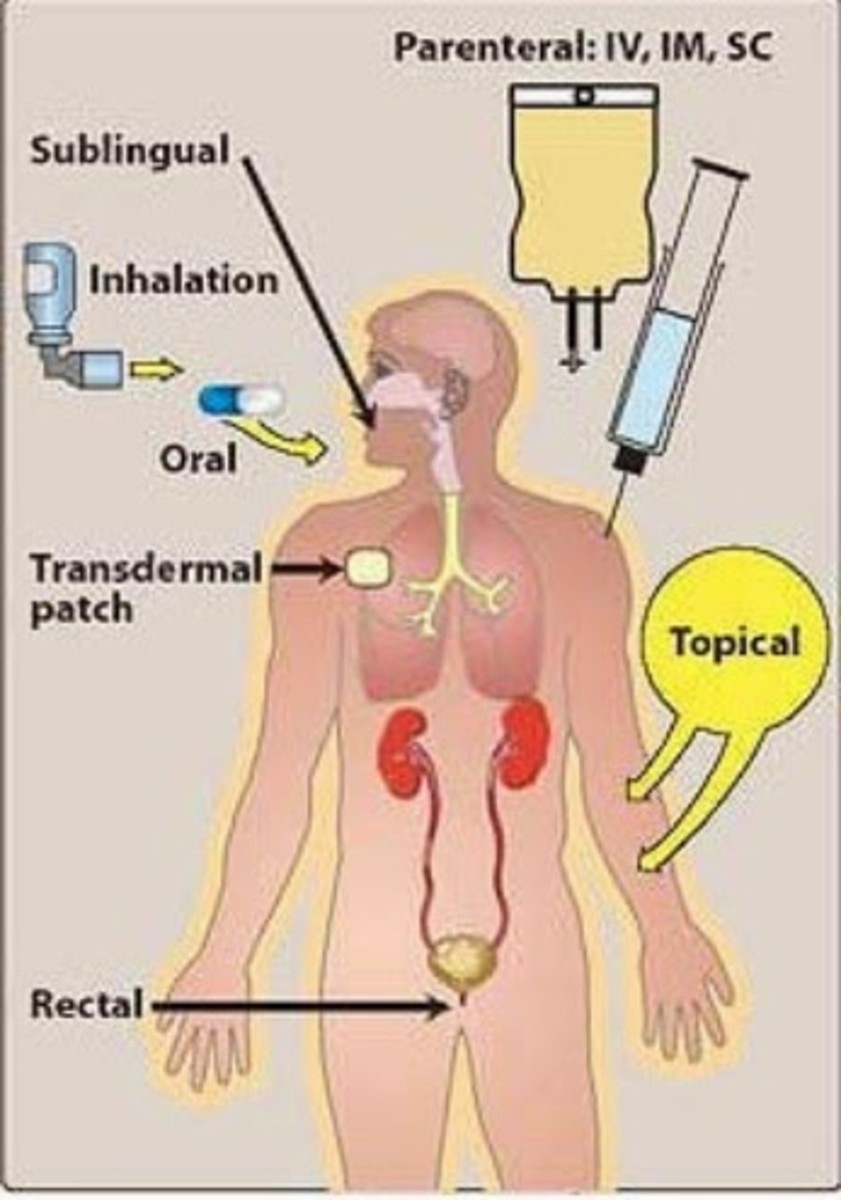
In-house studies control various research elements such as the diet of volunteers, often to see if a drug is fat soluble or whether there are side effects if it is taken on an empty stomach, or after a meal. The CRO will also document any side effects or complaints the volunteer mentions, no matter how trivial they might sound.
Phase One drug studies generally include compensation for the volunteer's time, and great care is taken to ensure there is no coercion in the recruiting or consent process.
Expert Guide to Pharmacological Research
What Do You Think?
Would you volunteer for a drug research study?
How Drugs are Tested in Patients With Diseases
After Phase One studies are conducted, the safety information about the drug or device is evaluated to see if the formulation or device can advance to Phase Two and Three research. In these two stages, the drug (or device) will be tested patients who have the disease or disorder for which it is being developed.
Contract Research Organizations usually work with physicians who are qualified to do research in these stages rather than conducting research on patient populations at their own sites.
The reason for this is that, with the hundreds of possible uses for a drug (depending on the disease, disorder, age of the patient, etc.), it would not be feasible to conduct all research at a contracted site.
The CRO will identify and contract with physicians across the country who practice in the therapeutic area for which the drug is targeted.
These physicians then identify patients who might be appropriate volunteers to test the drug or device.
There are many variables in recruiting appropriate patient volunteers. The physician is required to inform the patient of alternatives to the research, and to make certain there is no promise of effectiveness.
Some patients may not be showing a good response to usual therapies for their condition, and some may have rare diseases, for which there are few options for treatment.
It is the responsibility of the CRO to monitor physician sites to ensure records are kept and that the protocols of the drug research are followed.
Even OTC and Generic Drugs are Tested
Testing Over-the-Counter and Generic Drugs
Even if a drug is offered 'over-the-counter,' without a prescription, it must generally be approved for use in humans.
Some CRO testing is done of very common things you buy in the drugstore, such as aspirin and other pain relievers, and even sunscreen.
In some cases, the testing is done to allow the manufacturer to advertise the drub or product has shown a certain effectiveness in 'clinical trials.' At other times, it can be to compare it to another product, or test it with a new coating that makes it dissolve slower or eases stomach discomfort.
Some drugs are tested to be 'generic' versions of medications already on the market. After a drug's patent expires (around 16 years or so after it was first tested in humans), other firms can duplicate a formulation and sell it, but they must demonstrate their product performs exactly the same way as the original drug.
Many people feel drugs are overpriced - and to be sure, they are expensive. But when you consider that a drug can cost around $1 billion to go through all stages of research and get approval, and that it can take around 10 years for that to happen in some cases, it's easy to see why our prescriptions are so costly.