What is an Atomic Clock and How Does it Work?

You’ve probably heard of atomic clocks – those extremely accurate clocks that scientists use for mysterious reasons. You might also know that atomic clocks are extremely important to scientific experiments and the Global Positioning System.
To understand exactly what an atomic clock is and how it works, we first need to understand the general principles behind clocks. We all know that a clock's job is to keep track of the passage of time. The way in which clocks actually do this is something we tend not to think about. However, it turns out that all clocks, regardless of their mechanism, work on the same principle: they all keep time by counting the "ticks" of a resonator.
Tick tock
In a pendulum clock, the resonator is a pendulum (who'd have thought it?). The gears within the clock keep track of time by counting the ticks (resonations) of the pendulum i.e. the swinging to and fro. The pendulum usually resonates (or ticks) at a frequency of one swing per second.
A digital clock such as an alarm clock tends to use the oscillations on the mains power line (in Britain, that’s 50 cycles per second, a frequency of 50 Hertz. In the USA, it’s 60 cycles per second) as the resonator. So, in America, 60 oscillations would equate to one "tick" or resonation and one second would be counted.
Alternatively, a digital clock may use a quartz mechanism. This is an accurate mechanism which uses a specially manufactured quartz crystal as the resonator. Digital clocks count the resonations with digital counters, hence the name.
The important thing to take from this is that the accuracy of the clock is determined by the accuracy of the resonator at the given frequency. If the resonator does not resonate at exactly the same frequency all the time, the clock will lose or gain time.
That seems simple enough. But how does an atomic clock measure time?
Like all other clocks, an atomic clock counts the "ticks" of a resonator. An atomic clock uses as its resonator the resonance frequencies of atoms. The resonator is "regulated by the frequency of the microwave electromagnetic radiation emitted or absorbed by the quantum transition (energy change) of an atom or molecule." – Encyclopaedia Britannica.
That sounds a little confusing, so let me try to explain exactly what it means:
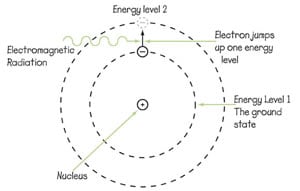
When an atom is exposed to electromagnetic (EM) radiation, such as ultraviolet rays or radio waves, the electrons orbiting an atom's nucleus can "jump" to higher energy states, then fall back down to lower energy states (see the picture on the right for a diagram of the process). Clocks based on this jumping of electrons can provide an extremely precise way to count seconds.
In fact, the international standard for the length of one second has long been based on atoms. Ever since 1967, a second has been officially defined as 9,192,631,770 cycles of the radiation that causes an atom of the element caesium-133 to vibrate between two energy states.
But how does it actually count the seconds?
Inside a caesium atomic clock, caesium atoms are funnelled down a tube where they are bombarded by radio waves. If the frequency of these radio waves is just right – 9,192,631,770 cycles per second – then the caesium atoms will "resonate" and their electrons will change their energy level.
A detector at the far end of the tube keeps track of how many atoms reaching it have changed their energy states. The closer the radio wave frequency is to 9,192,631,770 cycles per second, the more caesium atoms will reach the detector.
The detector gathers information about the number of higher energy atoms that have arrived and feeds this back to the radio wave generator. The generator adjusts its intensity accordingly. If too many caesium atoms have changed their energy states, the intensity of radiation produced by the generator will be reduced. If there are too few, it will increase accordingly. This synchronises the frequency of the radio waves with the peak number of caesium atoms striking it. Other electronics in the atomic clock count this frequency, and a second is counted when the frequency count is met.
Not all atomic clocks work in exactly the same way, as more accurate mechanisms are discovered over time. However, the basic principle is the same for all of them.

So, atomic clocks are good, right?
The basic advantage of an atomic clock is that atoms resonate at extremely consistent frequencies.
If you take any atom of caesium and use EM radiation to make it resonate, it will resonate at precisely the same frequency as any other atom of caesium, anywhere and anytime. That kind of accuracy is very different from the accuracy of a quartz clock. The quartz crystal in a quartz clock is manufactured in such a way that its oscillating frequency matches some given frequency - it doesn't matter what this frequency is, so long as the digital counters know what it is and are properly adjusted. But production methods aren't perfect, meaning that every crystal has a slightly different oscillating frequency, and things like temperature variations will change the frequency. A caesium atom always resonates at exactly same frequency - that's what makes atomic clocks so precise.
And when I say precise, I mean really precise. Many institutions maintain atomic clocks, and some of the most accurate in the world are at the National Institute of Standards and Technology (NIST) in Colorado. The NIST-F1 Caesium-133 atomic clock has an error of about 0.038 nanoseconds per day. That means that it would take around 100 million years to lose 1 second!
If you think that's impressive, you might like to know that scientists are currently working on an aluminium atomic clock. Such a clock would lose or gain a second every 3 billion years. That's more than half the current age of the sun!
Well, there you go. I hope I've given you a basic understanding of what exactly an atomic clock is and how it works.
Thanks for taking the time to read this hub. I hope you found it interesting. If you have any questions or feedback, please feel free to leave a comment below.