commercial production of recombinant proteins
Recombinant proteins
Recombinant proteins are encoded by modified genes using recombinant DNAtechnology. Recombinant proteins with modified gene sequence are used in commercial products manufacturing such as pharmacological products because their properties and structures are altered as compared to naturally occurring proteins. Recombinant proteins are being produced in yeast, filamentous fungi, bacteria, mammalian cells, insect cells, transgenic plants and transgenic animals. Vectors are specialized vehicles which are important for expression of recombinant DNA in host cells.
Applications of recombinant proteins:-
Recombinant proteins have their applications in life sciences, biotechnology and pharmacology. They are being used as diagnostic tools, as vaccines, therapeutic proteins and as functional enzymes. Over 150 human and veterinary based proteins have been approved so far and many more are being used as recombinant antibodies. They are being used in structural biology, biochemical experimenting and for industrial usage.
The various applications of recombinant proteins can be listed as follow:
- 1. In structural studies; crystallography of proteins and structural determination through NMR technique
- 2. Functional studies
- 3. Protein proteins interaction
- 4. Protein ligand interactions
- 5. Rational drug design target protein
- 6. Therapeutics proteins
Recombinant proteins are an outcome of expression of recombinant DNA in host cells:-
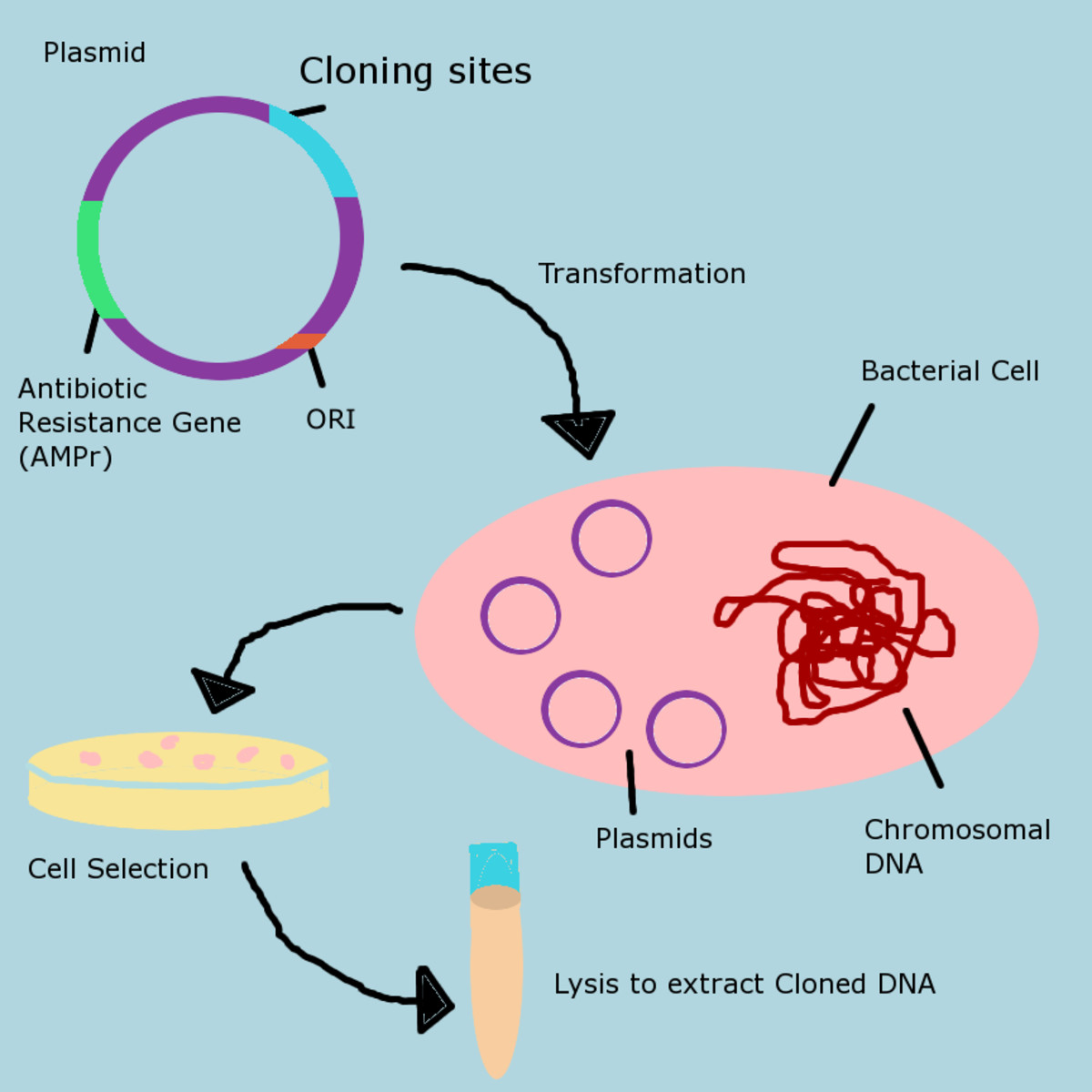
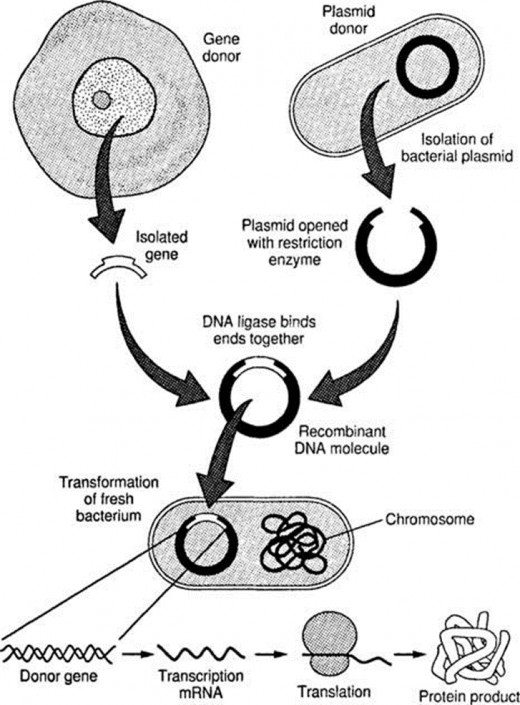
Using recombinant DNA technology, an organism can be induced to produce protein which it does not normally produced by it. The process is accomplished by inserting foreign gene of interest in its genome.
The genes which are being used in DNA technology are attained from host cells known as gene libraries. A collection of cell embracing a specific gene are contained within a gene library. For example E. coli cells can have genes for human insulin in their chromosomes and thus can be stored.
Steps in producing recombinant DNA:-
Taking E.coli expression system as an example,
- The gene of interest is cut from DNA molecule using a restriction enzyme.
- A bacterial plasmid is cut and then isolated making use of the same restriction enzyme.
- Next step is known as ligation during which plasmid and gene of interest are joined using enzyme DNA ligase.
- The plasmid is then incorporated back into bacterial cell.
Bacteria reproduce and required gene is cloned for production of specific protein.
Methods for production of recombinant proteins:-
Two methods are used for production of recombinant proteins.
- Molecular cloning
- Polymerase chain reaction
The difference between cloning and PCR is that, in cloning DNA replication is done in living host whereas in PCR it is done in test tube without living host.
Commercial production of recombinant proteins:-
Over the past two decades the demand for production of recombinant proteins has been increasing profoundly. With many known applications of recombinant proteins in different capacities especially in therapeutics, the commercial production of recombinant proteins is hugely preferred.
New and improved proteins are always in demand.
Proficient methodologies for recombinant proteins production are gaining importance as large number of applications requiring high quality proteins reach the market. To get a commercially viable products, there should be higher production efficiencies and low cost of final product.
Protein production systems:-
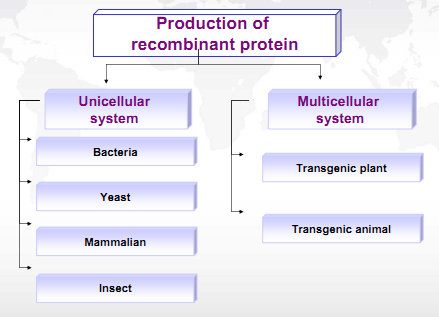
Different expression systems are being used for commercial production of recombinant proteins such as yeast, E. coli, insect cells and mammalian cells. Mammalian cell lines have been widely used for production of Therapeutics proteins as they have advantages over microbial systems because their cellular machinery is adapted to secretion of Recombinant products and to post translational modification such as glycosylation which is customary in many of the recombinant proteins in market.
Expression vector
The use of expression vector maximize gene expression.
Host
Minimizes turnover of gene product.
Choice of expression system
It depends upon two factors;
1. The ultimate use of the product
2. Nature of the individual protein which has to be expressed.
For example if protein of interest needs any post translational modification then bacterial expression system will not be suitable and mammalian expression systems will be preferable.
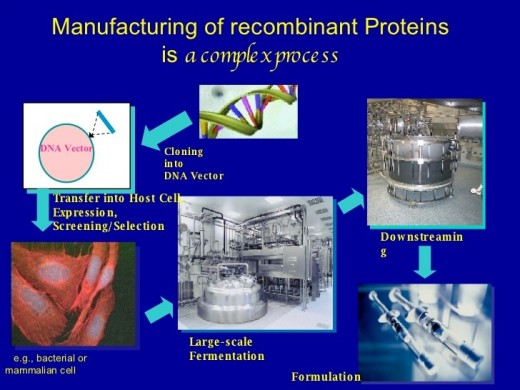
The manufacturing process of recombinant proteins:-
The manufacturing process of recombinant proteins consists of the following steps.
Step 1
Cell culture development
Cell bank: Production of cell line which is able to express protein of interest is done in this first step. Through Genetic engineering genetic stable strains are produced which can grow to high density in tank environment and are highly productive. Cells or strains are frozen in 100 of vials and stored.
Optimizing production: the strains grow expressing genes of interest. Cell culture processes are optimized to be precise and consistent at large scale. The most effective procedures are developed and protocol and parameters repeated optimizations is established to ensure upstream manufacturing process.
Step 2
Harvesting
Efficient methods for products separations are developed. Standardized centrifugation technology is being used for this purpose.
Step 3
Purification
Impurities are removed using difference in certain parameters between proteins and other molecules such as bio affinity, charge, hydrophobicity and molecular size. Sometimes downstream processes combines chromatography and filtration techniques in a sequence designed for operational simplicity and better performance.
A standardized platform or forming an assembly line for product can simplify the manufacturing process.
Step 4
Fill and finish
Ideal formulation; ideal formulation is determined for example antibodies must be stored in a stable environment for the product to remain potent. Liquid formulation is more preferable however powder formulation is also used but it is difficult to develop as compared to liquid formulation.
Step 5
Commercial scale production
During transferring of product to large scale manufacturing the processes can be scaled up to volumes in the order of 150000 liters. This scale up process is improved by multiple synergies (equipment, technologies, procedures, protocols).
Common problems encountered during production of recombinant proteins:-
Hundreds of recombinant proteins are being produced today on commercial scale but still there are challenges to overcome.
Loss of expression:-
Loss of expression may be observed due to structural changes in the recombinant gene or disappearance of the gene from the host cell. Loss of expression can be studied on three different locations of gene of interest,
- 1. In plasmids
- 2. Integrated into host chromosomes
- 3. Delivered by virus
Plasmid based system:-
Plasmids are molecular vehicle for recombinant proteins when using prokaryotes as host. Genetic manipulation of plasmid is also easy. Gene dose is higher when using plasmid as compared to when recombinant protein is integrated into chromosome. Gene dose depends upon plasmid copy number which may be few to 200. Plasmid impose a metabolic load on host cells using cellular recourses for replication, expression of encoded genes and for production of recombinant proteins. So when plasmid copy number increases, metabolic load increases. Consequently growth rate decreases which causes plasmid free rapid growing cells to dominate in the culture.
Plasmid loss is the main cause behind loss of expression in plasmid base systems. Plasmid instability due to structural instability, defective segregation during cell division due to plasmid multimerization can lead to loss of foreign gene expression.
Chromosomal integration:-
Adequate integration of foreign gene in chromosome is a time consuming and labor intensive process. It results in lower production rate as compared to plasmid based system. Another problem with chromosome integration system is that there may be possibility that gene of interest may be integrated into an inactive region of chromatin. Using locus control regions (LCRs) which ensures transcriptional regulation of trans gene, this problem is solved.
Viral vectors:-
Viral vectors are used for transporting gene of interest. The process using viral vector has two phases first cells are grown to desired density secondly they are infected with gene of interest. Limitation for using viral vectors is quality of viral stock. Serial in vitro passaging of stock can result in mutant viruses known as defecting interfering particle (DIP). The genome of DIP having several deletion replicates rapidly as compared to intact virus ,so DIP compete for cellular machinery decreasing yield of recombinant proteins. Time of infection and multiplicities of infection should be regulated too.
Post translational processes:-
1. Folding, aggregation and solubility
2. Proteolytic procession
3. Glycosylation
Folding, aggregation and solubility:-
Two molecules play important role in folding process.
Foldases accelerates the process, Chaperone prevent non-native insoluble folding intermediate formation. Cell stress by heat shock, nutrient depletion and other stimuli lead to misfolded protein which aggregate in intracellular aggregation known as inclusion bodies. Cell respond to stress by increasing production of various chaperone mainly of hsp70 and hsp100 family. When unfolded proteins accumulate intracellularly such as in endoplasmic reticulum, unfolded protein response is activated which leads to transcription of gene encoding for chaperons and foldases.
Over expression of heterologous protein results in inclusion body formation. They may reach non physiological concentration promoting aggregate formation.
Protein aggregation has been observed in bacteria, yeast, insects and mammalian cells. Aggregates protect proteins from proteolysis and assist in protein recovery by breaking the cell or inclusion bodies centrifugation. If production in inclusion bodies is preferred, solubilization and renaturation can be performed in different ways. However, the refolding step is an experimental process that on times is very inefficient, with yields typically lower than 10%. Thus, in many cases it may be problematic and expensive to attain a soluble functional protein after downstream operations. It is impossible to foretell whether a protein will aggregate or not in a particular expression system, or how easily it will be solubilized and renatured. Thus, a soluble protein is generally preferred. Numerous strategies have been anticipated for reducing protein aggregation. Several chaperones and foldases have been soundly cloned into hosts to facilitate protein folding. However, this strategy is not always successful. It is not possible to predict which chaperone will facilitate folding of a specific protein, or whether more than one chaperone or cofactor will be required. Overexpression of more than one chaperone has been explored with satisfactory results. Protein engineering can also reduce aggregation changing the extent of hydrophobic regions or using fusion proteins.
Proteolytic procession
Signal peptides are needed to transport protein to various cell compartments. They are then removed by signal peptidase complex which is bound to membrane of endoplasmic reticulum in eukaryotes and to cellular membrane of prokaryotes. Inefficient removal of signal peptide may lead to aggregation and retention in wrong compartment such as in endoplasmic reticulum. So yield of secreted protein decreases. Low signal peptidase activity can limit the production of recombinant proteins. To solve this problem, the E. coli signal peptidase I and the Bacillus subtilis signal peptidase have been overexpressed in E. coli and insect cells, respectively.
Low protease activity decreases the concentration of mature folded protein.
Another type of proteolytic processing is the removal of the N-terminal methionine.
This processing is performed by a methionine aminopeptidase (MAP) and occurs only in proteins in which the second amino acid is alanine, glycine, proline, serine, threonine or valine (35). Removal of N-terminal methionine is a common problem during expression by E. coli. Overexpression of recombinant proteins may saturate MAP. MAP has been overexpressed in E. coli to solve such a problem.
Glycosylation
It is a complex post translational process consisting of several steps and using tens of enzymes and substrates.
Protein stability, solubility, antigenicity, folding, localization, biological activity and circulation half life depends on it. It usually occurs in the endoplasmic reticulum and Golgi apparatus of eukaryotic cells, however N-glycosylation has been detected in proteins produced by bacteria. Factors affecting glycosylation are the presence of glycosidases either intracellularly or in the culture medium, synthesis of the dolicholphosphate oligosaccharide, which occur due to reduction in lipid pool, transport of sugar nucleotides to the endoplasmic reticulum or Golgi apparatus. Culture conditions can also affect glycosylation. For example, pH can affect the activity of extracellular glycosidases. The concentration of toxic byproducts, such as ammonia, CO2
, and hyperosmotic conditions, can reduce sialylation and the extent of
N- and O-glycosylation. Cell growth rate and protein production rate also influence
Glycosylation. Non authentic glycosylation trigger immune response in human and animal so authentic glycosylation process is necessary for a recombinant protein to be used as drug.
Transport and localization
Recombinant proteins may be directed to different cellular compartments by signal peptides or through fusion proteins. Different sites of protein localization have different advantages and disadvantages.
Protein localization is especially relevant when expressing recombinant proteins in
E. coli. Accumulation in the periplasm often results in soluble and correctly folded proteins, whereas cytoplasmic localization yields an inactive and insoluble product
Bioengineering approaches to solve common problem in heterologous gene expression
Recombinant proteins can be placed under variety of promoters which will decide whether gene expression is constitutive or inductive. When constitutive it may increase plasmid solubility as metabolic load of production still present so it is used when growth rate of host is not affected by gene expression.
When growth rate of cell is different from those of recombinant protein production rate then inductive expression is used which is done after particular cell density is reached. It expresses by induction of stimuli such as turning off or adding a nutrient which turn on a particular molecular machinery such as lac operon. Inductive system should be efficient economical and simple.
Growth control
Growth rate affects parameter which determines recombinant proteins accumulation rate. These parameters include percent of substrate utilized for cellular maintenance, RNA polymerase activity, ribosome number, plasmid stability, plasmid copy number, multimerization and distribution of cells in cell cycle phases.
So it is possible to control production through controlling some growth affecting factors which may be nutrients, concentration of dissolved oxygen and temperature.
Bioreactor and operational strategies
The function of bioreactor is control of environmental parameter in predetermined value beside containment. The number of parameter can be manipulated depending upon complexity of bioreactor. Among the conditions that can be controlled are oxygen, PH, temperature, agitation rate, redox potential, dissolved carbon dioxide, cell concentration, substrate concentration, volume, pressure, fluid dynamics and power input. The environment interact with several steps of recombinant protein production process namely cell growth, cellular metabolic rate, transcription, translation and post translational modification. From the importance of the environment, it can be seen that bioreactors have an enormous potential for increasing recombinant protein productivity
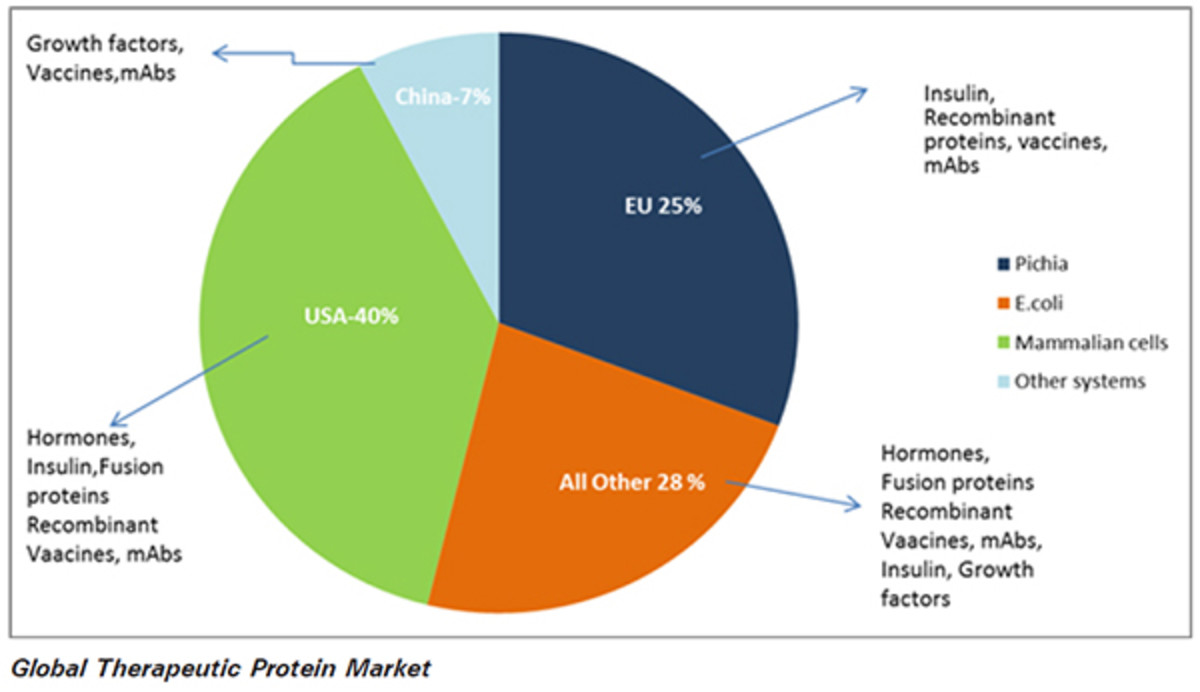
Recombinant Pharmaceutical proteins
In the past few years the market for the production of pharmaceutical proteins is growing rapidly. Since 1982 when first recombinant protein drug human insulin was discovered, it is advancing at a rapid rate. In 2008, 130 proteins were approved and now more than 170 proteins and peptides are in use.
The success of recombinant protein drugs over small molecular drugs is due to specific efficacy and lower number of side effects.
The recombinant DNA technology allows to choose expression system according to cost and need of modification in structure.
The clinical time for development and approval is shorter than small molecular drug and it also provide patent protection.
In 2003 global market of recombinant proteins drug was worth 37 billion dollars and reached to 90 billion dollars in 2010.
According to GBI report the market for recombinant therapeutics protein to 2017 is likely to grow and reach to 141.5 billion.
Today biopharmaceutics include recombinant hormones, hematopoietic growth factors, tumor necrosis factors, interleukins, interferons, blood clotting factors, thrombolytic drugs, enzymes, monoclonal anti bodies and vaccines.
Applications are diabetes, dwarfism, thrombocytopenia, myocardial infarction, multiple sclerosis, neutropenia, congestive heart failure, anemia, hepatitis, rheumatoid arthritis and cancer therapies.
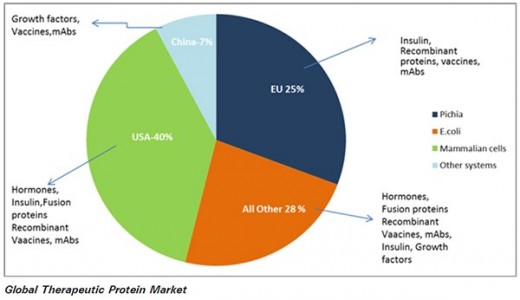
Recombinant proteins future perspective
Understanding how to facilitate expression of novel and difficult proteins through protein and cell line engineering will be essential to support future drug discovery. It is an absolute prerequisite for drug discovery that recombinant proteins can be produced in sufficient amounts and are of appropriate quality. The collection of novel biologics has expanded beyond classical antibodies into production of therapeutic proteins and complex antibody formats that pose fresh challenges for existing expression systems.