Strengthening Mechanisms of Metals
Cover Page

Introduction
Increasing the strength of materials is very important. What is strengthening mechanism? Metals are of great importance in the activity of man. There is no aspect of production that metal does not have role or roles to play before the final product is finally produced. Is there any that you can think of where metal have no role to play? Even as you are reading this article from your phone or computer, metals are performing some functions. The question is: how is metal performing function/functions as I am going through the piece of write-up? The answer is that either the internal or external or both parts of the device you are reading this piece from is made of metals, and without these metals, the device cannot perform any function effectively. In medicine and drugs production, the machines that are used for the production of the drugs are made of metals.
Strengthening mechanisms are processes of increasing the quality of metals by making the metals stronger so that they can be suitable for particular applications or usefulness. The reason why metals are alloyed is to increase the mechanical or the technological properties of the metals. The mechanical property that can be improved during the alloying process can be called strengthening of the metals. When strengths of metals are improved, the efficiency of the metals is increased. The reason why engineers carry out impact test on metals before using them for one particular application or the other is to know the strength of the metals before they are used for the function. If they do not do so, the building or construction can fail in later time because the strengths of the metals were not tested before use.
In the study of metallurgy, there are various mechanisms of strengthening of metallic materials. These mechanisms in some cases are dependent on the material or metals to be strengthened. The three major strengthening mechanisms to be discussed are grain size reduction, solid-solution alloying, and by strain-hardening. These three mechanisms will be explained in the subsequent sub-headings.
Check this topic from here
The Strengthening Mechanisms
What are the strengthening mechanisms in metallurgy?
Strengthening of metals reduces the dislocation motions of the metals, and hence makes the metals harder. When dislocation motions that take place in metals when the metals are under deformation are reduced, the strengths of the metals are increased and improved in return. Strengthening mechanisms in single-phase metals include the following:
- Grain-size reduction;
- Solid-Solution hardening; and
- Strain-hardening mechanism
Grain-Size Reduction
Have you ever wondered why some metals are harder than the other? Have you ever wondered why some metals are less ductile than the other? One of the reasons is because those that are harder and less ductile have reduced grains. When metals have smaller grains in them, the atoms are closely bonded and that property makes them harder and more resistant to ductility. Reducing the size of the grains that makes up any alloy is one of the means through which metals can be strengthened.
Let’s take for example that there are two groups of grains in a metal, say group A and Group B. These two groups are separated by a grain boundary. The grain boundary restricts dislocation motion between the two groups of grains for two major reasons:
- When group A wants to move into group B, there becomes difficulty for the movement to take place because they are of different direction. This eager to move to the other domain results to increase in crystallographic miss-orientation.
- When there is action of stress on the metals, atomic disorder takes place within the grain boundary region. This result to discontinuity of slip planes from one grain to the other. Slip planes are where dislocation motions take place or occur.
Also, it is a point to note that any metal with small grains have greater number of grain boundaries that coarse types, so it will be more difficult to break through these boundaries.
There is an important equation to be considered when it comes to strengthening mechanism by grain size reduction. The equation is given thus:
σy = σ0 + kyd-1/2
The above equation is called the Hall-Petch equation.
σy = Yield strength
d = Average grain diameter of the grain
σ0and ky are constants for a particular material.
The equation is not acceptable for both very large and extremely fine grains of polycrystalline materials. Grain size of metal can be reduced when the metal is its liquid state, by plastic deformation and by heat treatment. Boundaries between the grains prevent dislocation movement either by slip or by twin system.
Solid-Solution Strengthening
This process is also called solid-solution hardening. A solid-solution is solid mixtures which compose of a major metal and a minor metal that is uniformly distributed within the crystal lattice of the major. An alloy is an example of solid-solution.
Alloying with impurities strengthens and improves the hardness of metals. In the other words, solid-solution strengthening is the introduction of impurity into solid solution to improve the strength, hardness, and hinder dislocation motion in the metal. Example of impurity element in this context is nickel. It is used in copper to strengthen the copper. Why is pure metal softer than alloys? The reason is because the impurity elements introduced into the alloys strengthen them (the alloys). Alloys are stronger than pure metals because impurity atoms that go into solid solution ordinarily impose lattice strains on the surrounding host atoms.
The introduction of small impurity atoms into the lattice of host atom imposes tensile lattice strain on the host atoms. Conversely, introduction of large impurity atoms into the crystal lattice of host atoms imposes compressive strain on the host atoms (Materials Science and Engineering: An Introduction). When compressive strain is exerted, impurity atoms are adjacent to the dislocation line and above the slip plane.
You need this
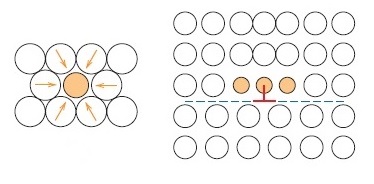
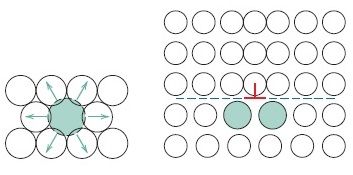
The slip dislocation will reduce with introduction of impurity atom and make plastic deformation difficult, hence enhance strength and hardness of the metal. Slip dislocation decrease because with the introduction of impurity atom, the strain of the crystal lattice will be decreased.
Strain-hardening
Strain-hardening is the phenomenon whereby a ductile metal becomes harder and stronger as it is plastically deformed. This process is also known as work hardening, cold hardening, or cold working. The reason for the name is because the process does not involve melting of metal (s). In the other words, it does not involve elevated temperature of the material to be hardened. Most non-heat treatable alloys are hardened using strain hardening.
A mathematical expression that is used during strain hardening is:
%CW = (A0 – Ad/ A0) * 100.
%CW is the percentage of cold working
A0 is the initial area of the metal cross section before plastic deformation.
Ad implies the area of the metal after deformation.
When a material like brass has an original strength or stress, σ0, acting on it and new stress of say σy, is reapplied on the same brass, its strength is increased. The alloy acquired the new improved strength because σy, is greater than σ0. Because the hardness of the metal is increased, dislocation motion in the alloy becomes retarded.
Dislocation motion in metals decrease with increase in cold working because there is high dislocation density taking place at that moment and the atoms collide to build stronger crystal lattice. That means when there are many "dislocations" in a crystal lattice, dislocation movement is hindered. The measure of the ability of any metal to be strain-hardened is known as strain-hardening exponent.
Conclusion
Discussed are the strengthening mechanisms of single-phase metals. They are used in making metals harder to fit into a particular function or functions. The three major strengthening mechanisms are grain-size reduction, strain-hardening, and solid-solution alloying.
References
- Material Science and Engineering: An Introduction by William D. Callister, Jr. and David G. Rethwisch
- Heat Treatment of Metals by Virjendra Singh
- Mechanical Properties of Metals by Donald Mclean