What is Light?

Throw a tennis ball on the wall and it bounces back, throw a ray of light on a sheet of glass and it passes through the glass as well as bounces back, what's wrong with this thing? some of it passes through a medium and some of it bounces back, what light actually is a question intriguing our minds from the last two centuries, many theories exists but neither of them has been fully successful in explaining the mysterious nature light.
Read on to uncover this interesting yet challenging mystery of light!
Particle model pictured light traveling as tiny particles of matter called corpuscles.
Evidence for Particle Nature of Light
Reflection - Newtons Views
Newton was of the view that light behaved as tiny particles of matter, this could easily explain reflection of light using Newtonian Mechanics and refraction by assuming that light being matter got attracted towards denser mediums and when it approached for example glass its speed increased. Later experiments however showed that light traveled slower in denser mediums and thus this view was abandoned by scientists in favor of the wave model only to be revived some years later in the form of photon model.
Wave model pictures light traveling as transverse electromagnetic waves.
Evidence for the Wave Nature of Light
Speed of Light
James Clarke Maxwell discovered that all electromagnetic radiation traveling through space traveled at a constant speed which happened to be the previously measured speed of light. This provided a very strong evidence that light was a form of electromagnetic radiation which travel in the form of transverse waves.
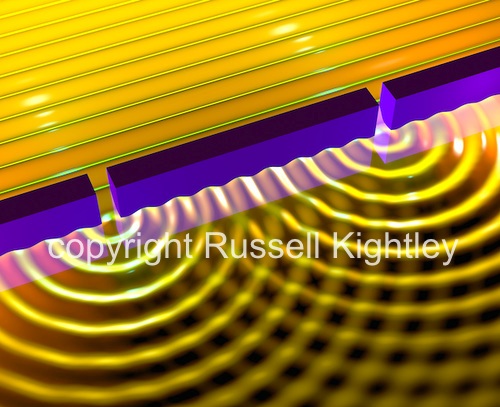
Interference and Diffraction
Thomas Young by means of his double slit experiment showed that light waves could interfere with each other constructively and destructively. This was shown by the pattern of light he obtained (pictured below) from this experiment where dark regions showed destructive interference and bright regions showed constructive interference.
Since interference is a property special to waves only it was postulated that light behaved as a transverse wave.

Photon model pictures light traveling in the form of 'photons' which are essentially packets or Quantum of electromagnetic energy (Quantum means a discrete value i.e all photons contain same amount of energy).
Evidence in favor of the Photon Model
Photoelectric Effect
Eisenstein argued that light behaved as particles called 'photons' on the basis of inferences he obtained from his photoelectric experiment.
Light of specific frequencies for made incident on different metal surfaces and it was found that this light when falls on metal surface causes electrons to be emitted. The following things were found after analyzing these emitted electrons.
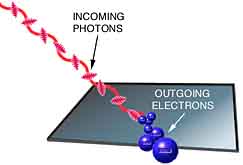
- Electron Emission starts immediately when light strikes.
- Emission occurs only above a certain frequency.
- Increasing frequency increases K.E of electrons.
- Increasing light intensity increases rate of electron emission.
The following table now shows why the Photon model proves to be more successful in explaining these phenomenon.
Observation
| Wave Model
| Photon Model
|
|---|---|---|
Low intensity light also causes photon emission.
| Weak light waves should have had no effect.
| Low intensity light means fewer photons, Not low-energy photons.
|
A minmum of threshhold frequency is required to initiate emmission
| Low frequency light should also have worked.
| Photon of low frequency light does not have enough energy to release an electron
|
Increasing frequency of light increases max K.E of emitted electrons
| Increasing Intensity should increase energy of electrons and not frequency
| High frequency means more energatic photons, so electrons gain more energy
|
Increasing Intensity of light increases the rate of electron emission but not energies of electrons
| More intensity should mean greater energy so more electrons released with more energy.
| Greater intensity means more photons per second, not more energetic photons so electrons cannot have more energy but rate of emission increases.
|
Emission of electrons starts as soon as light shines on metal
| Very intense light must be needed to have immediate effect.
| Single photon is enough to release one electron.
|
Why Photon Model is very successful in explaining the Photoelectric Effect?
How long will it take for the scientists to establish the true nature of light?
Conclusion - Wave Particle Duality
It is clear from the above discussion that in order to explain the photoelectric effect light must be pictured as particles and on the other hand wave model best explains interference and diffraction. How do we sort out this dilemma?
Scientists have to conclude that sometimes light behaves as waves and sometimes as particles, in particular:
- Light interacts with matter as a particle-the photon.
- Light travels in space as waves.
So, Is light a particle? or is it a Wave?, Scientists have come to terms with the dual nature of light or the wave-particle duality whilst the true picture being that the complete comprehension of the nature of light is a mystery modern science has yet to solve.....
"It seems as though we must use sometimes the one theory and sometimes the other, while at times we may use either. We are faced with a new kind of difficulty. We have two contradictory pictures of reality; separately neither of them fully explains the phenomena of light, but together they do".[1]
― Albert Einstein
© 2014 StormsHalted