What are Acids?
The pH Scale
Acids and Bases - Why are Acids Acidic?
We may not give acids and bases much thought in our daily lives, but they play a huge role in our daily lives. Plants need the correct pH to grow properly, acid rain can devastate entire ecosystems, and most of our synthetic fertilisers are made through neutralisation reactions between acids and bases. But what makes one thing acidic and another thing basic? And why are these substances so dangerous?
There are many ways to define acids and bases:
- An acid turns blue litmus paper red; a base turns red litmus paper blue
- An acid is a substance with a pH of less than 7; a base is a substance with a pH greater than 7
- An acid contains free hydrogen ions (H+); a base contains free hydroxide ions (OH-)
- An acid is a proton (H+) donor; a base is a proton acceptor
All of the above definitions are true, to a greater or lesser extent, but it is the last one that makes them dangerous. Hydrogen ions (also called protons) are constantly looking to balance out their positive charge, which makes them very reactive.
Certain concentrated acids (and some concentrated bases) are so reactive they can damage other materials, including living tissue. Acids that can damage the skin are called 'corrosive;' Bases that can damage the skin are called 'caustic'*
*The mechanisms by which acids and bases damage the skin are different, so scientists describe with different words.
What is the Difference Between a Base and an Alkali
Most pupils I teach are familiar with the term acid, but give it's opposite as 'alkali.' Whilst it is true that alkalis have a pH greater than 7, contain hydroxide ions and accept protons, there are plenty of substances that are not alkali that do the same.
An alkali is a soluble base - it is a base that dissolves readily in water. Make sure to remember:
- The opposite of an acid is a base;
- A base that dissolves in water is known as an alkali;
- All alkalis are bases, but not all bases are alkali.
How to Measure pH - Make Red Cabbage Indicator!
What does pH mean?
Often, the term pH is used to say how strong an acid or alkali is. pH actually has nothing to do with whether an acid is strong or weak. The letters 'pH' stand for power of hydrogen! The scale tells us the concentration of hydrogen ions in the solution.
- At pH 0, there are lots of hydrogen ions - the solution is very acidic
- at pH 7, there are equal numbers of hydrogen ions and hydroxide ions - the solution is neutral
- At pH 14, there are lots of hydroxide ions - the solution is very basic (Alkaline if it dissolves in water)
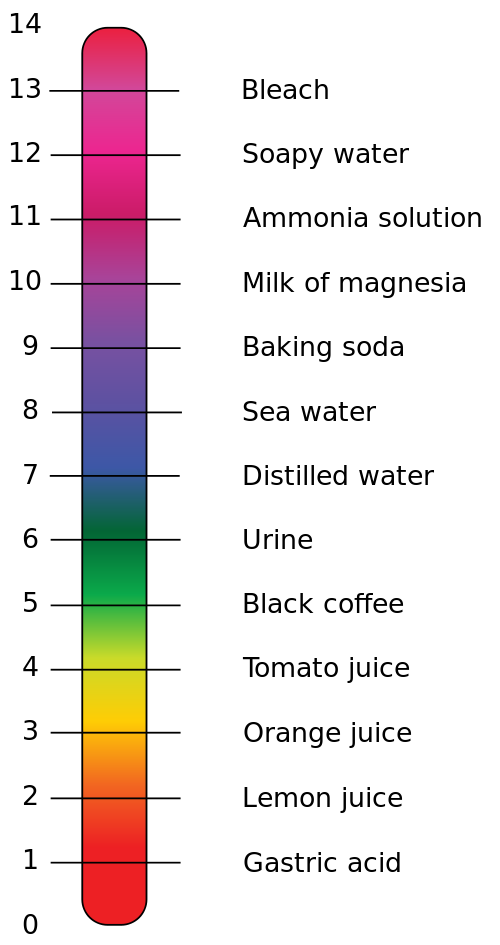
pH Scale Examples
pH
| Substance
| Information
|
|---|---|---|
0
| Hydrofluoric Acid
| Highly corrosive. Acts as a catalyst in oil refining
|
1
| Sulphuric Acid
| Used in the production of fertilisers. Also found in acid rain
|
2
| Lemon Juice
| Contains around 5% citric acid, a weak acid which gives lemons their sour taste
|
3
| Cola drink
| Contains phosphoric acid
|
4
| Beer
| The Carbon dioxide bubbles lower the pH of beer slightly
|
5
| Black Coffee
| The acidity of coffee depends on the altitude at which the coffee beans were grown
|
6
| Cow's milk
| Cow's milk sours due to bacteria producing lactic acid
|
7
| Distilled Water
| When the number of positive and negative ions balance, you get neutral pH. Most water contains low levels of ions so is not neutral.
|
8
| Baking Soda
| A gentle cleaning product due to it's slightly raised pH
|
9
| Toothpaste
| Acid can damage tooth enamel, so a slight base is added to regulate the pH
|
10
| Milk of Magnesia
| Neutralises excess acid in the stomach
|
11
| Ammonia
| Used in many cleaning products, as well as the production of fertilisers
|
12
| Bleach
| Contains sodium hypochlorite to help break down those tough stains
|
13
| Heavy Duty Oven Cleaner
| These need to be very caustic to break down thick grease and fat
|
14
| Sodium Hydroxide
| Also known as Caustic Soda
|
An acid donates hydrogen ions when in solution. The higher the concentration of hydrogen ions released, the stronger the acid. Bases accept hydrogen ions in solution. They also contain ions of the opposite charge, such as hydroxide ions.
Strong Acids and Bases
So if pH has nothing to do with how strong an acid or base is, what makes an acid strong or weak?
We already know that acids form hydrogen ions when in water, and that they 'donate' these ions. This is known as ionisation. The strength of an acid depends on how well it ionises:
Strong Acids (E.g. Hydrochloric Acid)
Strong acids completely ionise in water
Strong acid → Hydrogen Ions + other ions
There are lots of hydrogen ions and very few acid molecules (remember it is the hydrogen ion that makes an acid reactive). In strong acids, the H+ ion concentration is higher and the collision frequency greater
Weak Acids (E.g. Ethanoic Acid)
Weak acids only partially ionise in water. The set up a reversible reaction
Weak acid ↔ hydrogen ions + other ions
An equilibrium mixture is formed with lots of acid molecules, but not many hydrogen ions. In weak acids the H+ ion concentration is lower and so the collision frequency is lower.
Remember! The strength of an acid or base is down to how well it ionises (how easily the hydrogen or hydroxide ions dissociate from the rest of the acid molecule), not it's pH