Accutane (isotretinoin) - Will it be Pulled from the Market?
What is Accutane?
Isotretinoin (Accutane) is a drug originally designed to treat brain and pancreatic cancer as well as other forms of cancer. Accutane (isotretinoin) is still used for this for purpose as it is a non-selective systemic (body-wide) agent capable of arresting the growth of rapdily dividing cells.
Isotretinoin is an old drug as drugs go, having been discovered in the 1930s. It is also marketed under the names:
- Amnesteem (Mylan)
- Claravis (Barr)
- Clarus (PREMPHARM)
- Decutan (Actavis)
- Isotane (Pacific Pharmaceuticals)
- Izotek (BlauFarma)
- Oratane (Genepharm Australasia)
- Roaccutane (Roche Pharmaceuticals)
- Sotret (Ranbaxy)
- Isotrex or Isotrexin (Roche Pharmaceuticals)
How it Came About
Originally, high doses of fat soluble vitamin A could be used to treat severe cases of acne. These doses tended to reduce the production of sebum, an oily substance produced around hair follicles.
The doses recommended for this treatment were upwards of 500,000 IU of vitamin A per day. However, such high doses are toxic.
Hoffmann-La Roche Pharmaceuticals found a derivative of vitamin A which is called isotretinoin; they gave it the brand name Accutane. This derivative was first marketed in 1982.
Rouche made two hundred million dollars in sales per year until the patent expired in 2002. That's a potential total return of four billion dollars.
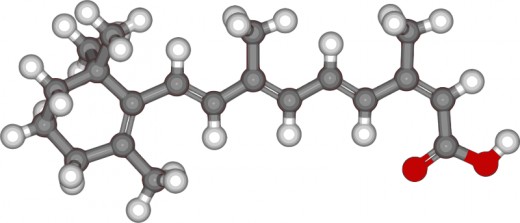
Is Accutane on it's way Out?
Roche Holding (formerly Roche Pharmaceuticals) began pulling it's version of isotretinoin off the shelves in mid June 2009. This amid early warnings that drug is linked to
inflammatory bowel disease (IBD). The last week of October 2009, a study was published that gave those risks a number. The study found that users of Accutane have almost twice the risk of developing IBD as non-users. Considering all of the other problems with Accutane this may represent the "last nail in the coffin" of this popular, yet controversial drug.
Though Roche has pulled it's stocks, generic versions are still available. However, this latest research could lead to a complete withdrawal of isotretinoin in all it's forms.
In it's twenty-seven (27) year history the drug has been found to cause serious birth-defects, increases the risk of depression, suicidal thoughts, and the various other side-effects listed below.
Side Effects (Toxicity)
Common side-effects to isotretoinoin include:
- Increased sensitivity to the sun
- Joint pain
- Muscle pain
- Headaches
- Thinning hair, permanent
- Thinning skin, permanent
- Elevated cholesterol
- Liver toxicity
- Nose bleeds
- Pink-eye
- Rosacea
- Back pain
Rare, but serious side-effects include:
- Impaired night vision
- Cataracts
- Optic neuritis (inflammation of the optic nerve)
- Menstrual disturbances
- Inflammatory bowel disease
- Pancreatitis (inflammation of the pancreas)
- Hepatitis (injury to the liver)
- Corneal opacity
- Papilloedema (increased pressure on the optic disc [where the nerve connects])
- Idiopathic intracranial hypertension (increased pressure within the skull)
- Skeletal hyperostosis (overgrowth of bone)
- Psychosis
Clinical Use
Accutane is only recommended when other acne treatments fail. A course of treatment typically begins with topical (on the skin) medications such as benzoyl peroxide, followed by a course of antibiotics (oral/and or injected), and finally Isotretinoin.
Because of the toxicity dangers, isotretinoin is a closely monitored course of treatment and therefore more expensive due to more frequent doctor visits and blood serum level tests. The medicine itself is also expensive with cost per dose rising incrementally by milligram.
As a drug, isotretoinoin is a cumulative drug, in other words it must build up in the blood-stream to be effective. This is another factor in cost as repeated blood-serum level tests must be performed to attain 120–150 milligrams per kilogram. (mg/kg).
Because it's a cumulative effect drug Isotretoinoin must be administered, usually twice daily, for three to six months. At the end of the three to six months patients may be required to begin the regimen again if severe acne recurs in the one to two month hiatus after the end of the fist course of treatment.
Birth Defects
Isotretinoin is also linked to a high level of birth defects. More common types of birth defect including hearing and visual impairment, missing earlobes, facial dysmorphism, and mental retardation.
Between 1982 and 2003 two thousand (2,000) women became pregnant while on Isotretinoin therapy with most pregnancies ending in abortion or miscarriage. Of the two thousand, one hundred sixty babies were born with birth defects.
In response the Food and Drug Administration began a campaign called iPLEDGE.
iPLEDGE is a web-site/program started in March 2006 and run by the FDA. Before a patient can be prescribed Accutane (or any other form of Isotretinoin) health-care providers and their patients must register on the web-site before a course of treatment can begin.
Alternative Treatments
First and foremost, since Accutane (and it's generic variants) are prescribed for serious cases of acne the following may not produce the desired results.
However, my article on Natural and Technological Treatments for Acne does offer some vastly safer alternatives to drugs such as Accutane.
Heat and light have both been found to be effective against the bacteria that causes acne and food items commonly found in most kitchens are also somewhat effective against acne. Both these technologies and methods are covered in the hub (link above) mentioned.
Coda
With all of these side-effects, including such a high potential for birth defects, the only other drug with as many controls is thalidomide. Because of this some former patients and consumer advocates wonder why Accutane is still on the market.
Dr. Steven Stone may hold the answer: "It would be a true disaster if this medication would become unavailable. People don't die of acne, so it's easy to say, 'This is a drug that causes inflammatory bowel disease; let's take if off the market.' But that ignores the psychological harm of severe acne."
Disclaimer
The author was not compensated in any way, monetarily, with discounts, or freebies by any of the companies mentioned.
Though the author does make a small profit for the word count of this article none of that comes directly from the manufacturers mentioned. The author also stands to make a small profit from advertising attached to this article.
The author has no control over either the advertising or the contents of those ads.