AMA Asks the Fed to "Reclassify Marijuana"
Cannabis Origins
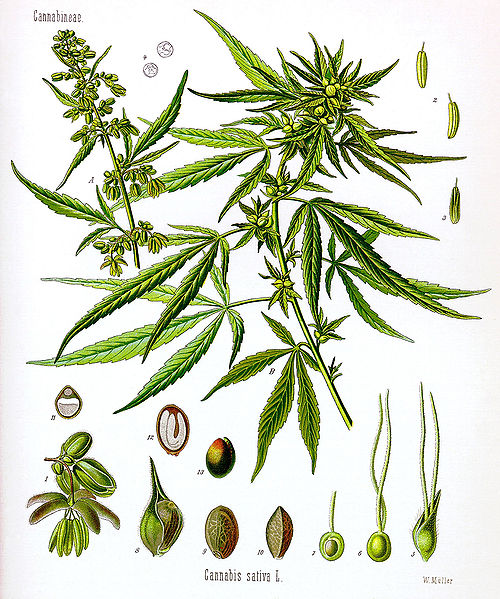
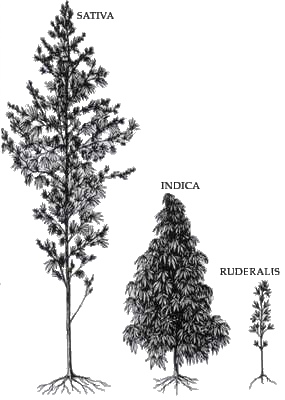
The three cultivars (domesticated weeds) are Cannabis Sativa, Cannabis Indica, and Cannabis Ruderalis. All three are certain to have originated in Central & South Asia.
It is thought that Cannabis Sativa was originally cultivated for it's long soft fibers. These fibers were used for the production of rope, paper, and cloth. In this use Cannabis Sativa is called Hemp. Pottery shards in China show the distinct impression of hemp fiber. These shards date to 5,000 B.C. demonstrating that hemp has had a long history of practical use in rise of civilizations.



Cannabis Sativa
Hemp was a popular crop in the United States both before and after independence. George Washington and Thomas Jefferson both cultivated hemp. Benjamin Franklin started the first American paper-mill which made paper exclusively with hemp fiber. The first draft of the U.S. Constitution was written on hemp paper.
The psychotropic substances in hemp are minuscule.
Hemp was a popular fiber in WWII (see Hemp for Victory video below) with uniforms, canvas, and rope the primary products produced from it's fibers. Hemp plantations were centered mainly in the Midwest and Kentucky. Hemp can also be used for oil (bio-fuel, paint vehicle, face creams, cooking oil, plastics, and as food supplement) and as a food product in the form of seeds. Seeds can be eaten raw, ground into meal, sprouted (malted), made into hemp milk (similar to soy milk), prepared as tea, and used in baking.
Hemp for Victory

Cannabis Indica
Cannabis Indica was found to have many more psychotropic substances (though at the time the effect was known, but not the mechanism) than Cannabis Sativa. Many pharmacopoeia catalogs of the early twentieth century listed it as an ingredient in medicinal preparations. Cannabis Indica extract was used for menstrual cramps, headaches, poor appetite, and pain relief.
Studies in the twenty-first century link THC, the active ingredient in cannabis, to improving the appetites and general outlook of AIDS and chemotherapy patients. Other studies have indicated that the cannabis psychotropic compound THC helps Alzheimer's Disease (AD) patients deal with agitation and anxiety by reducing both. It has also been shown to reduce arterial blockages.
Other reports have linked THC use with reduced tics in people with obsessive compulsive disorder and Tourette syndrome.
Federal (United States) Law
As of now, the Food and Drug Administration (FDA) acknowledges that "there has been considerable interest in its use for the treatment of a number of conditions, including glaucoma, AIDS wasting, neuropathic pain, treatment of spasticity associated with multiple sclerosis, and chemotherapy-induced nausea...", but the agency has not approved the use of marijuana for medical purposes.
However, the FDA also stated: "marijuana has a high potential for abuse, has no currently accepted medical use in treatment in the United States, and has a lack of accepted safety for use under medical supervision."
The Controlled Substances Act passed in 1970, classifies marijuana is a Schedule I drug. This is the strictest classification of drug which also includes heroin, LSD and Ecstasy. In 2005 the Supreme Court ruled that the Commerce Clause of the U.S. Constitution allows the federal government to ban the use of cannabis for any purpose. This includes Cannabis Sativa as hemp.
The CSA of 1970 makes acquiring marijuana for research difficult. The National Institute on Drug Abuse is the only legal federal entity allowed to both grow and dispense marijuana for use or research. In the mast the NIDA has refused researchers cannabis for study, often after long delays. Additionally, the NIDA has refused to supply cannabis of a specific strength citing safety concerns.
"Most clinical studies have been conducted using cannabis cigarettes with a potency of 2-4% THC. However, it is anticipated that there will be requests for cannabis cigarettes with a higher potency or with other mixes of cannabinoids. For example, NIDA has received a request for cigarettes with an 8% potency. The subcommittee notes that very little is known about the clinical pharmacology of this higher potency. Thus, while NIDA research has provided a large body of literature related to the clinical pharmacology of cannabis, research is still needed to establish the safety of new dosage forms and new formulations." - source NIDA Advisory Council
AMA Asks for Reclassification
Stated Reasons
On November 10, 2009, the American Medical Association (AMA) urged the federal government to
reconsider its classification of marijuana as a dangerous drug and to reconsider its declaration that cannabis has no
accepted medical use.
This is a "sea-change" in the AMAs attitude toward marijuana. The call for policy change is a significant shift from the AMAs position, starting in 1997, that marijuana should remain on the list of Schedule I drugs. The call for change puts the association behind other calls for more research on the plant.
The AMA consists of about two hundred fifty thousand (250,000) physicians. In changing it's policy the group's stated goal is to clear the way for clinical research, help create cannabis based medicines, and devise less harmful ways to deliver the drug.
State Approval of Medical Marijuana
The decision, during an annual meeting in Houston, highlights an ongoing change in the view of cannabis. In the report the AMA points out that marijuana was once linked to what the federal government called "homicidal
mania." With California and twelve other states approving the use of cannabis for medical use, the drug's reputation has moved steadily from a recreation only problem (once reputed to cause madness in it's users) to a drug that has beneficial effects for the chronically ill. Another twelve states are considering legalizing cannabis for medical use this year.
American Medical Association Basis
Dr. Edward Langston says: "Despite more than 30 years of clinical research, only a small number
of randomized, controlled trials have been conducted on smoked
cannabis. [These studies are] insufficient to satisfy the current
standards for a prescription drug product."
In the written policy the AMA also states: "This should not be viewed as an endorsement of state-based medical cannabis programs, the legalization of marijuana, or that scientific evidence on the therapeutic use of cannabis meets the current standards for a prescription drug product."
The AMA also rejected an amendment to the proposal which stated: "smoking is an inherently unsafe
delivery method for any therapeutic agent, and therefore smoked
marijuana should not be recommended for medical use" though all doctors will readily admit that burning something and inhaling the smoke is not good for your lungs.
Despite the seeming "newness" of this position the AMA also rejected calls to make the drug illegal in 1937 citing numerous uses in medicines.
Obama Administration Changes
This year, (2009) the white house ordered federal narcotics agents to concentrate on illicit marijuana production and sale and leave medicinal users who followed state law alone. The gist of this order is that pursuing medical marijuana users was a waste of Drug Enforcement Administration (DEA) resources.
However the office of the White House drug czar, under Gil Kerlikowske, stressed the administration's opposition to legalization. In that statement the office deferred to "the FDA's judgment that the raw marijuana plant cannot meet the standards for identity, strength, quality, purity, packaging and labeling required of medicine."
Marijuana Advocates Speak Out
Medicinal cannabis advocates welcomed the development. "They're
clearly taking an open-minded stance and acknowledging that the
evidence warrants a review. That is very big," said Bruce Mirken of The Marijuana Policy Project. "It's not surprising that
they are moving cautiously and one step at a time, but this is still a
very significant change."
American College of Physicians
In 2008, the American College of Physicians, the
second-largest physician group behind the AMA, called for "rigorous scientific
evaluation of the potential therapeutic benefits of medical marijuana"
and an "evidence-based review of marijuana's status as a Schedule I
controlled substance."
California Medical Association
Last month, the California Medical Association passed resolutions calling the criminalization of cannabis "a failed public health
policy." It called on its members to take part in ongoing debate for changing public policy.
Disclaimer
The author was not compensated monetarily, via discount or freebies for the contents of this article. The author has no professional or casual connection to the AMA, ACP, CMA, or MPP nor has the author been compensated in any way, shape, or form, but cannabis growers or their representatives.