Anemia & Colon Cancer
CASE STUDY
Mrs KH, 65 year old 55kg woman with Chest Pain and Shortness of Breath on exertion. Feeling progressively tired and losing weight for approximately 6 months. Taken to hospital for investigation.
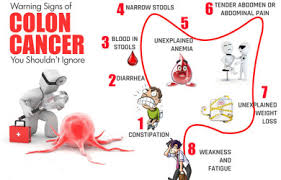
*Presenting condition: Very Pale, Lethargy, Appetite Loss, Constipation (past 2 weeks)
*No Medical History or medications
*Social/Family History: Lives alone, Father died of Colorectal Cancer
*Symptoms: Nausea, colicky abdominal pain and Chest tightness
*98% Oxygen saturation, Small bilateral pleural effusion (otherwise clear), normal temperature, pale conjunctiva, soft tender abdomen
Lab results Overview [1]:
-Low Hb(49g/L): Hemoglobin is the protein that carries oxygen. Low levels indicate anaemia, bleeding, malnutrition, cirrhosis or cancer [1]
-Low RCC: Also known as Red Cell count.[2] It refers to the estimate of the amount of red blood cells per litre of blood.[3] Low levels indicate anaemia from blood loss, bone marrow failure, malnutrition (e.g. iron deficiency), overhydration or mechanical damage to red blood cells.[3]
-Low HCT : Hematocrit is defined as the amount of space in blood occupied by red blood cells.[1] Decreased in anaemia, bleeding, malnutrition, cirrhosis and cancer.[1]
-Low MCV: Mean corpuscle volume refers to average size of red blood cells. Abnormal levels may indicate anaemia, thalassemia and malnutrition.[1]
-Low MCH: Mean corpuscle haemoglobin refers to the average amount of haemoglobin in each red blood cell.[1] Abnormal levels may suggest anaemia, thalassemia or malnutrition.[1]
-High Reticulocytes: may suggest hemorrhage/hemolysis.[4] Usually increased when there’s increased in red cell production. [4] Since levels are not normal, the diagnosis is not anaemia of chronic disease.[4]
-Low Iron (<4micromol/L): refers to low amount of iron that is bound to transferrin [2]
-Low Ferritin: Ferritin is the primary storage form of iron in the body.[2] Low levels are best indication for iron deficiency anaemia.[2] Low levels are treated with an iron supplement or multivitamin.[2]
-High Transferritin/transferrin: these are iron-binding plasma glycoproteins that control levels of free iron in bodily fluids. [3] High levels may indicate iron deficiency anaemia, pregnancy, oral contraceptive use.[4]
-Elevated CEA : is a tumour marker especially relevant for cancers of the gastrointestinal tract.[5]
Her liver and kidney function appear normal.
Diagnosis: Colon Cancer confirmed, Anaemia of Chronic Disease
Treatment: Referral to Oncology for staging and treatment
Initial Hospital Management Plan
Pallor in skin and conjunctiva, fatigue/lethargy, muscle weakness, chest pain, shortness of breath are some symptoms of anaemia.[3] Normal temperature excludes infection.
Weight loss, abdominal pain and bowel disorders like constipation and a family history of colorectal cancer could indicate gastrointestinal malignancy though more tests are needed to make diagnosis definitive rather than rely on these symptoms alone.[6] However, in this case, tissue sample of colonoscopy have confirmed colon cancer.
Ferritin is the best single blood parameter to diagnose iron deficiency and levels lower than 30mcg/L without inflammation (assuming normal CRP levels) confirm iron deficiency anaemia (IDA).[6] Other blood markers of IDA are decreased Hb (lower than 120g/L in women) with hypochromic and microcytic erythrocytes, low serum iron and high levels of transferritin.[6,7]
IDA is caused by compromised erythropoiesis when iron stores are low or empty.[6] IDA is usually related to malignancy in the gastrointestinal tract especially in colon cancer.[6]
Anaemia in critical illness is normally treated with red blood cell (RBC) infusions.[1] Any cause of iron deficiency anaemia must be treated in a targeted fashion and iron supplementation should be initiated.[1,6] Treatment goals are to normalise Hb and replenish iron stores.[6]
According to the WHAS 11 year study (The Women’s Health and Aging Study), hemoglobin levels <13g/dL (i.e.<130g/L) in home-dwelling women aged 65 and above is an independent risk factor for mortality and disability.[7] Since Hb and iron levels in KH are dangerously low, rapid increase of iron stores is needed and hence intravenous iron is preferred over oral supplementation.[6] Ferric carboxymaltose isomaltoside and iron are administered at 20mg/kg body weight over fifteen to sixty minutes.[6] Furthermore, we do not want to worsen her constipation which can result from oral iron use.[6]
Her total iron requirements can be calculated as follows[6]:
Total iron needs (in mg) = 3.84 x weight x (desired haemoglobin-measured haemoglobin) + storage-iron
We should measure reticulocytes after 1 week of iron supplementation and we want levels to increase.[6] Iron treatment should continue three months after Hb levels have normalized.[6]
The presence of IDA (due to blood losses) and anaemia of chronic disorders (due to cancer, inflammation or infection) is applicable here.[8] So measures have to be taken to stop any active bleeding or hemorrhage (e.g. thermocoagulation, platelets, surgery[8a]) while parenteral iron and erythropoiesis-stimulating agents are also given to correct blood stores which is especially important if chemotherapy is commenced because of chemotherapy-induced anaemia.[8] A meta-analysis of controlled trials by Petrelli et al. and systematic reviews and meta analysis of randomized controlled trials by Gavter-Gvili et al. showed that parenteral iron decreased risks of transfusions by 23% and increased hemotopoietic response by 29% compared to if erythropoiesis-stimulating agents alone as well as reduce the need for transfusions without affecting mortality or adverse events.[8]
The confirmed diagnosis of colon cancer warrants the need for further staging and management in oncology which can include surgery and possible chemotherapy and radiotherapy which all can cause further anaemia. [8]
Calleja et al. reported a retrospective, multicentre observational, non-interventional two consecutive blind cohort comparison of using ferric carboxymaltose to various oral forms of iron in colon cancer patients (Inclusion criteria: Hb<12-13, Ferritin<30mcg/L, TSAT<20%) where 111 patients received intravenous iron versus 155 did not.[8] The objective was to evaluate efficacy of pre-operative intravenous iron in colon cancer patients with IDS.[8] A statistically significant reduction in the need for transfusion was reported in those receiving intravenous iron (9.9% versus 38.7%, p<0.001). [8] Also reported significantly improved haemoglobin levels in those given intravenous iron as well as lower incidence of re-interventions, post-surgery complications and shorter hospitalization.[8] Blind studies are good evidence to study effectiveness of an intervention.
Using intravenous iron pre-surgery in anaemic patients reported significant reduction in transfusion and improved Hb levels (1.5 versus 0.5 in controls) by Calleja et al. but no benefit of improved haemoglobin levels was observed in those receiving intravenous iron post-operatively after colon cancer surgery as reported by Titos-Arcos et al. [8]
Hedenus et al. reported a small, open-label single centre prospective randomized study comparing the use of intravenous ferric carboxymaltose 1g versus control group (no iron given) in 17 patients with functional iron deficiency and lymphoid malignancies undergoing chemotherapy and reported a significantly higher increase of Hb(>1g/dL) levels compared to control group (p=0.02) without causing- treatment-related adverse events.[8]
An observational prospective study done over three months by Toledano et al. investigated the clinical use of median dose of 1 g ferric carboxymaltose in 367 patients with malignancies or solid tumours and reported median haemoglobin levels improvements from 10.3 to 11.8g/dL and further reported that this increase in Hb is the same in those treated with iron monotherapy or with additional erythropoiesis-stimulating agents (34% of patients).[9] No adverse reactions or hypersensitivity were reported.[9]
The causes of anaemia in cancer include reduced production of red blood cells (RBCs) from nutritional deficiencies, lack of RBCs production due to chronic disease, diminished response to erythropoietin (EPO), bone marrow infiltration by tumour or bone marrow suppression due to chemotherapy, radiotherapy or surgery plus increased RBCs lost from blood loss attributed to tumour, surgery or hemolysis.[10]
In patients >50years old with significant anaemia and family history of colorectal cancer like in KH, both upper and lower GI investigation is necessary even when celiac disease is not found. [11]
Management of IDA required iron supplementation. [11] Blood transfusions are only indicated for those with or at risk of cardiovascular instability because of the degree of anaemia.[11] According to KH’s results, her oxygen saturation results, blood pressure and heart rate and ECG are normal and indicate cardiac stability.
Post-operative anaemia is a common problem in colorectal cancer.[12] It can be caused by multiple factors like the malignancy itself, blood loss in surgery, inflammatory response to surgery with functional iron deficit.[12] This needs to be considered since KH will most possibly needs to undergo surgery to remove polyps. [12]
A prospective, randomized and placebo-controlled study (anaemic patients with Hb <8.9g/dL; n=52) investigated using a combination r-HuEPO (recombinant human erythropoietin) and intravenous iron in treated (Group 1) versus untreated control group(Group 2).[12]
Group 1 (n=26) : Given 100mg IV sucrose hydroxide iron (ferritin) three times a week and 30000UI of subcutaneous r-HuEPO twice a week for 14 days
Group 2 (n =26) = Control Group
It reported that the combination resulted in significant increases in baseline Hb 14 days after treatment in the post-operative period in colorectal patients, therefore reducing the need for blood transfusion (p=0.001).[12] Summary of findings are indicated by the average Hb levels as follows:
On 7th Day of treatment: Group 1: 10.6 + 0.2g/dL versus Group 2: 9.2 + 0.4g/dL
On 14th Day of treatment: Group1: 12.2 +0.3g/dL and Group 2: 10.1+0.3g/dL
This confirms statistical difference (defined as p<0.05 in this study) between the two groups in haemoglobin levels (t=-16.1,p=0.001 on the 7th day and t=-3.23,p=0.001) after treatment.[12] Blood transfusion was used when Hb was <8g/dL.[12] Further findings showed the number of patients receiving blood transfusions are zero (from Group 1) versus 10 (in Group 2) who received total of 15 blood units (38.46%) p<0.05.[12]

TRALI-Definition
As per prospective observational study at two US academic medical centres, TRALI (transfusion-related acute lung injury) and possible TRALI are defined as hypoxia and bilateral pulmonary oedema which occurs during or within six hours of a blood transfusion that is not a result of other causes such as intravascular volume overload or heart failure with presence of bilateral opacities on chest radiographs. [13,14,15]
TRALI occurs in 1 in 5000 Units of Plasma containing products (FPP, Platelets or Whole Blood) and leads to increased nosocomial infection risks and death.[13]
Since ten years ago, evidence has confirmed that TRALI is often because of transfusion of white blood cell antibodies to patients who are at risk.[15] TRALI can be both antibody-mediated and non-antibody mediated .[15] Evidence however do not support that all TRALI is caused by transfusion and rather can also be due to acute respiratory distress syndrome(ARDS) being the causative risk factor therefore categorizing this new class of patients as “transfused acute respiratory distress syndrome” rather than “possible TRALI”.[9] So there are 2 categories namely TRALI (which is transfusion- related) and Possible TRALI (which is due to ARDS).[15]
KH’s risk of TRALI:
Blood transfusions present risks like iron overload, procedural issues, viral and bacterial infections, direct immune injury and immune modulation.[9] In colorectal cancer, data from a meta-analysis reported a higher rate of recurrence in those who received RBC transfusions and a higher rate of infections.[9] An overall negative effect on the end point of cancer recurrence and overall mortality was observed in people who received RBC transfusions in those with colorectal, head and neck, prostate, breast and gastric cancers.[9] Although most studies in Toledano et al. included retrospective analyses, the choice to give RBC transfusions and the risks versus benefits have to be carefully considered especially in patients with solid tumours intended to be treated in the aims of curing.[1,9 ] It is advised that for most critically ill patients, Hb levels of 70-90g/L is safe and tolerable.[1] If there’s no bleeding, Hb level of 70-80g/L is a trigger for transfusion and target Hb levels are 70-90g/L.[1] It suggests that in non-bleeding individuals, to transfuse one single unit of red cells and remeasure haemoglobin levels before considering transfusing more.[1]
According to all data from a prospective, two-centre multiyear study in 2012 by Toy et al., TRALI risk is dependent on transfusion dose during or within the first six hours of onset of acute lung injury or ARDS.[15] However there was contrasting data from the same study that the risk of possible TRALI did not increase with dose of units transfused.[15] In conclusion, they confirmed that TRALI risk is transfusion related whereas “Possible TRALI” was not.[15]
No definitive data or investigations into the Possible TRALI cases in humans as a result of transfusion due to factors released by stored platelets were available hence it is premature to conclude that Possible TRALI is not transfusion-related.[15] A prospective clinical study however investigated antibody and non-antibody transfusion related factors including plateletpheresis units storage time and evidence showed that transfusion factors like longer storage time of platelets did not increase TRALI risks in humans.[15] No studies have definitively supported the benefits of non-antibody transfusion factors in human TRALI.[15]
With KH’s predisposing ARDS risk factors (presence of small bilateral pleural effusion, respiratory symptoms like shortness of breath and chest pain and tightness), my concern is that this may place her at increased sensitive risk whereby even transfusion of one unit causes TRALI.[15]
Two randomized controlled trials were then conducted to address the above concerns to assess whether transfusion of one unit of unwashed stored RBCs cause lung impairment in intensive care unit and surgical patients at risk for TRALI and confirmed that there was no impairment of lung function in either groups.[15] Peters and colleagues also reported in a randomized controlled trial that one unit transfusion of 35-day stored autologous RBCs in presence of endotoxemia (which is an ARDS risk factor) does not cause human lung injury.[15]
Diagnosis was made by an expert panel within the study by Toy et al.2012 to 2015 and concluded that Possible TRALI is a diagnostic term only to be used when the causative factor was the progression of the patient’s underlying ARDS risk factor after reviewing the 12-hour clinical course before transfusion.[15] The mortality and morbidity of Possible TRALI closely resembles ARDS from any cause rather than the more benign course of TRALI.[15]
Although additional evidence in another study was found against the increased risks of TRALI associated with transfused antibody (evidenced by no increased rates of TRALI in anti-HLA-positive compared to anti-HLA-negative plasma-rich blood components), considering that KH is likely to undergo surgery and cancer therapy (chemotherapy, radiation etc), we want to minimise the infection and other risks involved in blood transfusions if transfusions are indicated and required.[9,15]
Ways to minimise TRALI risks
Toy et al.2012 reported that plasma from female donors was a dose-dependent risk factor for TRALI but not Possible TRALI because of the presence of certain white blood cell antibodies more commonly found in female donors.[15] In later years of this study, it confirmed that male-predominant plasma transfusion method helped reduce the incidence of TRALI but not Possible TRALI.[15,16 ]
TRALI occurs acutely (<24 hours immunological post-transfusion reaction) due to transfusion of unfractionated plasma-containing blood components like platelets, red cells and plasma.[17]The pathogenesis theory behind TRALI is widely thought to be a result of the passive transfer of human leucocyte antigen (HLA) or human neutrophil antigen (HNA) antibodies found in donor’s plasma that directs against recipient’s leucocyte antigen.[17] This antibody-antigen reaction activates neutrophils within the microcirculation of the lung, releases oxidases and proteases which cause blood vessel damage and leakage.[17] Furthermore, accumulation of biological response modifiers like biologically active lipids can occur and induce TRALI in susceptible people.[17] In general, TRALI is precipitated by anti-HNA class I and class II antibodies and less commonly due to anti-HNA antibodies.[18]
To reduce risks of antibody-mediated TRALI, donors with HLA or HNA antibodies (due to pregnancy history or transfusion) are not used for plasma products or apheresis platelets.[17] Strategies updated by The Australian Red Cross include using Male-only blood or plasma for manufacturing FFP or cryoprecipitate and to collect Apheresis platelets only from male and female donors who have never been pregnant.[1,17]
Other methods include implementing a better coordination and hemovigilance program at a National level to determine true incidence and types of adverse events related to transfusion and better policies made to minimize transfusion related adverse reactions.[16]
Adhering to current guidelines for using blood components especially for plasma helps decrease TRALI risk and a restrictive transfusion method has been linked to a decreased TRALI incidence compared to a liberal transfusion strategy.[19] Clifford et al. also reported greater incidence of TRALI and Possible TRALI in perioperative patients in those transfused with greater volumes of blood.[19] Moreover, the largest randomized trial TRICC comparing different haemoglobin transfusions triggers reported that using red cells more restrictively had similar efficacy to more liberal methods and less mortality and less organ dysfunction and cardiovascular complications.[20]
Hence, optimal transfusion techniques of using enough blood products to maximise clinical benefit and outcomes without adverse events like TRALI is the goal. [19]
3) Mrs KH adamantly refused to receive blood transfusions or any blood products based on her religious beliefs. What other treatment options are available for her? Select the most appropriate option for the short term management of KH’s anaemia.
The main goal is to normalize haemoglobin levels and restore iron levels.[6] It is important to address the three main factors that cause anaemia in cancer patients other than cancer therapy itself which are iron deficiency, cytokine surge and reduced erythropoietin.[21]
Kalyani et al. reported that cancer-related anaemia is not due to insufficient erythropoietin or iron levels but rather the improper EPO response.[20] More studies are needed to analyse the relation between EPO and TNF-α as a pathway to treatment.[21]
In a study of anaemic patients undergoing major cancer surgery with postoperative anaemia by Duffen et al. (a prospective double-blinded study using a two-tailed student t-tests) reported higher Hb increases at weeks 3, 4, 6 and 8 weeks after using a single dose intravenous iron isomaltoside (20mg/kg) to replace iron as soon as patient is stable post-operatively and as soon as possible after operation versus control (no treatment group) (p<0.05). [22]
In general, treatment options for anaemia in cancer patients like KH includes red blood cell transfusions, ESAs(erythropoiesis-stimulating agents) and iron supplementation.[23] The main aim is to improve quality of life while minimizing the number of blood transfusions to reduce transfusion-related risks.[23] Since KH refuses to receive blood or blood-related products like recombinant human erythropoietin, her options are iron and ESAs.[23]
However, for patients undergoing chemotherapy and radiation, the favourable response to ESAs range from 35% to 70% and efficacy is limited by iron deficiency, existing infections, neoplastic activity and poor bone marrow reserve.[23] Moreover, some meta-analyses and studies have suggested the increased risk of thromboembolic events and mortality with ESAs especially in those not on chemotherapy.[23]
Iron deficiency is classed as absolute (depleted iron reserves) or functional (normal iron reserves or increased).[23] Functional deficiency is the predominant cause of IDA in cancer and KH’s Ferritin levels 1 mcg/L which is >100ng/L (or >0.1mcg/L) confirms this diagnosis assuming there’s increased Hepcidin levels in KH too.[22] Although iron reserves may be sufficient in functional IDA, there’s lack of bioavailable iron especially if treatment of ESAs (erythropoiesis stimulating agents) are given that further increase red blood cell production and iron usage.[23]
Supported by the National Comprehensive Cancer Network, intravenous iron monotherapy to reduce number of transfusions is suggested for:
*Absolute IDA (ferritin<30ng/L or <0.03mcg/L) .[23]
*In patients on ESAs with ferritin between 30 and 800ng/L (or 0.03 to 0.08mcg/L) and transferring saturation of between 20% and 50% for Functional IDA . [23]
The European Organization for the research and treatment of cancer recommends iron replacement for absolute and functional IDA and The European Society for Medical Oncology reports that intravenous iron replenishment in IDA increases Hb and lessens transfusion need.[23]
Although KH’s Ferritin levels = 1 mcg/L, solid evidence from this and previous discussed studies supports that intravenous iron supplementation would still benefit her both pre-surgery and post-surgery.[8,9,11,12, 23] Oral iron is ineffective in cancer patients because of reduced intestinal absorption (95% lost in faeces) and the small amount that’s absorbed is stuck within the enterocytes because of metabolic disorders induced by inflammatory cytokines (especially interleukin-6) which reduce iron availability further through increasing hepcidin concentrations which cause a block to iron input into the circulation. [23] Intravenous iron overcomes this absorptive inflammatory iron block because injected form is directly received by macrophages.[2] The best is the stable complex ferric carboxymaltose (FCM) which provides a slow prolonged iron release allowing for a high dose iron infusion administration while eliminating the risk of excessive iron being released in its free or toxic forms (which is of particular importance in cancer to prevent further progression). [22] Dose is 1000mg given in a single fifteen-minute infusion. [23]
Supported by meta-analyses published so far, using ESAs and intravenous iron versus ESA alone causes significant increases in Hb with a more rapid effect, better quality of life, decreased need for transfusion and reduced doses of ESA required for therapy (thus more cost effective).[23] Treating chemotherapy-induced anaemia by FCM is more clinically beneficial than ESA alone.[23,24]
Using intravenous FCM as monotherapy in comparison to FCM with ESAs was investigated in an observational study (n=639 solid tumour and onco-hematological patients) and reported similar increases in Hb levels (1.4g/dL and 1.6g/dL respectively).[23] Using FCM alone pre-surgery in colorectal cancer also reduced direct and indirect costs of hospitalization and reduce transfusion need compared to oral or less stable iron complexes like saccharate iron.[23]
In light of studies into the risks of ESAs in cancer, it is important to determine staging of KH’s cancer and whether treatment goal is curative or palliative so as to direct the best treatment options.[24] In malignancy with a curative intent, ESAs risk of thromboembolism and possible death direct the minimization of using ESA.[24] ESA use as per FDA 2007 guidelines should be limited to those receiving chemotherapy for palliative intent.[24] Six randomized trials and some additional studies reported adverse health events with ESA use in cancer patients like decreased survival, increased tumour progression or recurrence and increased risk of thromboembolic events thus restricting its use in cancer patients.[24]
In presence of renal insufficiency in cancer, ESAs are used in rare circumstances and clinicians should always be guided by risks versus benefits.[24] The change in FDA 2008 guidelines to contraindicate ESA use in cancer patients receiving chemotherapy for curative intent is supported by data from eight randomized trials and one meta-analysis of RCTs along with new published similar data from RCTs investigating mortality differences with ESA use.[24]
KH should be advised of risks and benefits of any therapy to allow for informed decision and written consent as follows [24]:
-Medication information outlining risks versus benefits
-Goal of ESA is to reduce RBC transfusion requirements
- FDA guidelines (2008) indicated ESAs should be avoided in cancer patients with intent to cure
-Harms and benefits of ESAs versus RBC transfusions
-ESAs found to shorten overall survival or speed of tumour growth in some cancer patients
-Adverse effects associated with ESAs: Blood clots
-ESAs are not advised for cancer patients not on chemotherapy or are on radiotherapy because of increased fatality
-ESA may improve fatigue or quality of life in some but main goal is to reduce transfusion requirements
Before deciding to use ESAs, a thorough history, physical examination and diagnostic tests are recommended to confirm alternative causes of anaemia not due to chemotherapy or underlying malignancy-related hematopoietic issue.[24]
Despite using erythropoietic agents, immunomodulatory drugs and medicines that target inflammation and correct anaemia, they unfortunately don’t improve cancer control or survival.[23] The main aim in cancer patients is not to just eliminate anaemia but to determine the cause of anaemia and treat appropriately.[23]
Anemia causes and management post-discharge
Even though Ferritin levels help diagnose IDA, I will require additional information to determine a differential diagnosis of type of IDA anaemia cause such as [23]:
-Hepcidin levels; if increased suggests functional IDA. [23]
-% Transferrin saturation ; needs to be <20% to be Functional IDA. [23]
-To determine alternative casues of anaemia, need to investigate if anaemia is Microcytic (IDA), Macrocytic (Cobalamin deficiency) or Normocytic (both IDA and Cobalamin deficiencies).[7]
If results show normocytic red blood cells, renal insufficiency is suspected in low erythropoietin levels when creatinine clearance is <60mL/min.[7] Kidney function is advisable to address whether or not this is a cause of anaemia as well.[24]
Serum EPO, Iron and TNF-α help to precisely diagnose the cause of anaemia in cancer patients like KH, therefore allowing for most appropriate treatment to improve quality of life.[21]
The staging of her cancer and whether the goal is curative or palliative will determine the best treatment options.[23,24]
Elevated CEA:
Elevated CEA is a tumour marker for both benign and malignant cancers especially of the gastrointestinal area (most common ones are colon and rectal cancers) though raised levels are observed in pancreas, stomach breast, lung and medullary cancer if the thyroid and ovarian cancers.[5]. A rising CEA level may indicate cancer progression or cancer recurrence but other test are required to confirm these.[5] CEA levels above 20ng/mL may indicate metastatic disease and with the concomitant presence of anaemia, further investigation into the metastasis of cancer to her lungs or any other areas is required to direct appropriate therapy.[5, 25] Chemotherapy can also elevate CEA as a result of death of tumour cells and CEA release into the bloodstream.[5]
Most likely cause of anaemia in KH:
Reduced red cell survival is common in critically ill patients like KH especially in presence of sepsis where red cells become deformed due to oxidative damage and changes in cell membrane.[20]
Please refer to my discussion of lab results on page 1 on this report. Elevated CEA is a tumour marker especially for gastrointestinal cancers (e.g.colon cancer).[5] Colon cancer/malignancy and related hemorrhage/hemolysis (elevated reticulocytes) and functional IDA is the most likely cause of her anaemia.[5,23]
Biochemical factors are vital in determining anaemia causes in cancer that is not due to inadequate iron or EPO but rather the presence of inflammatory cytokines.[21]
Functional IDA is the most prevalent cause of anaemia in cancer as revealed by blood tests levels of Ferritin and assuming increased Hepcidin levels and <20% Transferrin saturation.[23] The transport of iron is carried out by transferrin which supplies bone marrow and other tissues with iron as per demand.[23] Transferrin iron uptake originates from absorption by duodenal enterocytes or from recycled senescent erythrocytes by macrophages.[23] Hepcidin (a regulatory hormone in liver) controls intestinal absorption and macrophage release of iron and increased levels degrades the iron export protein (ferroportin), preventing iron availability to be transported.[23] Interleukin-6 (an inflammatory cytokine) also reduces iron availability through activating Hepcidin.[23] Therefore, even if iron reserves are sufficient, cancer patients may develop lack of bioavailable iron especially if treated with ESAs.[23]
Manage Anaemia post-discharge:
Assuming cancer chemotherapy with curative intent in KH, intravenous FCP has been supplied to KH to correct her anaemia in hospital.[23] Also assuming that surgery was successful to remove polyps and suspicious lesions, monitoring of cancer recurrence is imperative.[5] Assuming normocytic anemia, KH is to be supplemented with Cobalamin and injectable form of iron to bypass absorption and metabolic problems if Hb levels are still <13g/dL (i.e. <130g/L) [7] Other causes of anaemia in elderly like folate deficiency, renal insufficiency and hypothyroidism need to be investigated too to allow for optimal therapy for KH. [7]
Post discharge medications [5,26]:
*Vitamin B12 injection: Initial IM 1000mcg on alternate days for 1 to 2 weeks until improvement occurs then maintenance 1000mcg IM once a month
*Iron polymaltose (Ferrum-H): Dose according to manufacturer’s recommendations and guided by Hb levels
REFERENCES
Understanding the Complete Blood Count (CBC).2017. Available from: Bottom of Form
https://www.sonoraquest.com/test-information/understanding-the-complete-blood-count-cbc
2)Pandora320. Hematology/Oncology Flash Cards. 2014-12-11 07:05:14 https://www.freezingblue.com/flashcards/print_preview.cgi?cardsetID=282430
3)myDr.com.au.Full blood count.2001-2017.Available from: http://www.mydr.com.au/tests-investigations/full-blood-count-fbc
4)Wilkipedia.Transferrin. 2017 Mar 26. Available from: https://en.wikipedia.org/wiki/Transferrin
5) CEA Blood Test National Insitute of Health.Carcinoembryonic antigen (CEA). Available from: http://www.medicinenet.com/carcinoembryonic_antigen/article.htm
6) Jens Frederik Dahlerup, Martin Elvindson, Bent Ascanius Jacobsen, Nanna Martin Jensen, Soren Peter Jorgensen, Stig Borbjerg Laursen, Morten Rasmussen and Torben Nathan. Diagnosis and treatment of unexplained anemia with iron deficiency without overt bleeding.Dan Med J 2015;62(4):C5072
7)Lodovico Balducci, MD. Cancer-related Anemia: Special considerations in the Elderly.Oncology Journal 2007 Jan 1. Available from: http://www.cancernetwork.com/oncology-journal/cancer-related-anemia-special-considerations-elderly
8) Lebrun F, Klastersky J, Levacq D etal. Supprt Care Cancer. 2017 Apr 7. Available from: http://link.springer.com.ezproxy.utas.edu.au/article/10.1007/s00520-017-3672-1
8a) Barnert J and Messmann H. Diagnosis and management f lower gastrointestinal bleeding. Rev.Gastroenterol Hepatp;, 2009 Nov 6(11): 637-46. Available from: https://www-ncbi-nih-gov-ezproxy.utas.edu.au/pubmed/19881516
9) Toledano A, Luporsi E, Morere JF et al (2016) Clinical use of ferric carboxymaltose in patients with solid tumours or haematological malignancies in France. Support Care Cancer 24:67–75. doi:10.1007/s00520-015-2728-3.Available from: https://link.springer.com/article/10.1007/s00520-015-2728-3
10) Dirk Schrijvers. Management of Anemia in Cancer Patients: Transfusions. The Oncologist. 2011 Aug. Vol 16 Suppl3:12-18. Available from: http://theoncologist.alphamedpress.org/content/16/suppl_3/12.long
11)Goddard AF, James MW, McIntyre AS, Scott BB; British Society of Gastroenterology. Gast 2011 Oct;60(10):1309-16. Available from: https://www-ncbi-nlm-nih-gov-ezproxy.utas.edu.au/pubmed/21561874
12) Naco M, Gani H, Mandi A, Llukacaj A, Kodra N, Prifti P. Recombinant human erythropoietin (r-HuEPO) and intravenous iron for treatment of postoperative anemia in colorectal surgery. European journal of anaesthesiology 2012 VL: 29 PG: 97.XR: EMBASE 71084307.Available from:
http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/869/CN-01026869/frame.html
13)Shailaja J.Hayden, Tyler J.Albert, Timothy R.Watkins and Erik R.Swenson. Anemia in Critical Illness-Insights into etiology, consequences and management.Concise Clinical Review. Am J Resp Critical Care Med. 2012 May 15.Vol 185 Iss.10;pp1049-1057
14)Critical care compendium. TRALI.2017. Available from: https://lifeinthefastlane.com/ccc/trali/
15)Toy P, Kleinman SH, Looney MR. Proposed revised nomenclature for transfusion-related acute lung injury. Transfusion. 2017 Mar;57(3):709-713. doi: 10.1111/trf.13944. Epub 2016 Dec 26. Available from: http://onlinelibrary.wiley.com.ezproxy.utas.edu.au/doi/10.1111/trf.13944/full
16)Rahul Vasudev, Vijay Sawhney, Mitu Dogra, and Tilak Raj Raina Transfusion-related adverse reactions: From institutional hemovigilance effort to National Hemovigilance program. Asian J Transfus Sci. 2016 Jan-Jun; 10(1): 31–36.doi: 10.4103/0973-6247.175391.Available from: https://www-ncbi-nlm-nih-gov.ezproxy.utas.edu.au/pmc/articles/PMC4782490/
17) Australian Red Cross Blood Service. Transfusion-related Acute Lung Injury (TRALI).Australian Red Cross.2016 Nov 29. Available from: https://transfusion.com.au/adverse_transfusion_reactions/TRALI
18) Sofiane Tariket, Caroline Sut, Hind Hamzeh-Cognasse, Sandrine Laradi, Bruno Pozzetto, Olivier Garraud, and Fabrice Cognasse.Transfusion-related acute lung injury: transfusion, platelets and biological response modifiers.Expert Review of Hematology Vol. 9 , Iss. 5,2016 . Available from:
19) Jeongmin Kim and Sungwon Na. Transfusion-related acute lung injury: clinical perspectives. Korean J.Anaesthesiol 2015 Apr;68(2):101-105. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4384395/
20) Timothy s.Walsh, Duncan LA Wyncoll and Simon J stanworth. Managing anaemia in critifcally ill adults.BMJ Vol 341.c4408. 2010 September 11:Pages 547-551
21) P Kalyani, K Jamil. A study on biochemical facet of anemia in cancers: A strong link between erythropoietin and tumor necrosis factor alpha in anemic cancer patients .Indian Journal of Cancer. 2015 Vol 52(2):127-132. Available from: http://www.indianjcancer.com/article.asp?issn=0019-509X;year=2015;volume=52;issue=1;spage=127;epage=132;aulast=Kalyani
22) Duffen A, Rooms M, Raobaikady R. Postoperative intravenous iron therapy in the treatment of anaemia associated with major cancer surgery. CENTRAL 2016(10). Blackwell Publishing Ltd. Published 2015 Vol 70:Page 33
23)Flavio Augusto Naoum. Iron deficiency in cancer patients.Rev Bras Hematol Hemoter.2016 Oct-Dec;38(4):325-330. Published online 2016 Jun 22. Available from: https://www.ncbi.nih.gov/articles/PMC5119669/
24) Rizzo JD, Brouwers M, Hurley P, Seidenfeld J, Arcasoy MO, Spivak JL, Bennett CL, Bohlius J, Evanchuk D, Goode MJ, Jakubowski AA, Regan DH, Somerfield MR; American Society of Hematology and the American Society of Clinical Oncology Practice Guideline Update Committee.American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Blood. 2010 Nov 18;116(20):4045-59. doi: 10.1182/blood-2010-08-300541. Epub 2010 Oct 25.Available from: https://www.ncbi.nlm.nih.gov/pubmed/20974674
25) Vasa Jevremovic,1 Amer Abboud,2 and Stuart Krauss.Case Report:Colonic Metastasis with Anemia Leading to a Diagnosis of Primary Lung Adenocarcinoma. Case Reports in Oncological Medicine.Volume 2016 (2016), Article ID 5275043, 4 pages.http://dx.doi.org/10.1155/2016/5275043.Available from: https://www.hindawi.com/journals/crionm/2016/5275043/
26) Prof Nick Bickley, MD, Dr Helen Calabretto, RN, RM,PhD, Prof Chris Del Mar, MD, Dr John Dowden, MBBS (UK), Prof David Gordon,MBBS et al. Australian Medicines Handbook 2011. Adelaide SA 5000. Page 330-331