Clinical Evaluation of Bio-Oss: A Bovine-Derived Xenograft for the Treatment of Periodontal Osseous Defects in Humans.
Article summary; Richardson CR, Mellonig JT, Brunsvold MA, McDonnell HT, Cochran DL
Richardson CR, Mellonig JT, Brunsvold MA, McDonnell HT, Cochran DL: Clinical
evaluation of Bio-OssA: a bovine derived xenograft for the treatment of periodontal
osseous defects in humans. J Clin Periodontol 1999; 26: 421–428.
C Munksgaard, 1999
Abstract:
The purpose of this study was to compare the bovine derived xenograft (BDX) Bio-Oss to demineralized freeze dried bone allograft (DFDBA) in human intrabony defects.
The primary objectives were to determine the amount of reduction from baseline osseous defect depth parameters as a result of grafting with Bio-Oss, and determine if Bio-Oss will provide defect fill greater than or equal to that provided by demineralized freeze dried bone allograft. Secondary objectives were to determine if grafting vertical defects with Bio-Oss reduces probing depths and demonstrates a gain in clinical attachment around teeth after 6 months, and to identify any unusual clinical findings or complications seen with this material.
Study design:
17 healthy patients (7 males, 10 females; aged 34-67) with no systemic disease with moderate-severe periodontitis were treated. Surgically, defects were included only if the intraosseous defect depth was >3.0 mm. Final selection included 30 defects. The sites were randomly assigned treatment with DFDBA or BDX (single blind randomized controlled clinical study). Soft tissue and osseous defect measurements were taken the day of surgery and 6 months post-operatively at re-entry.
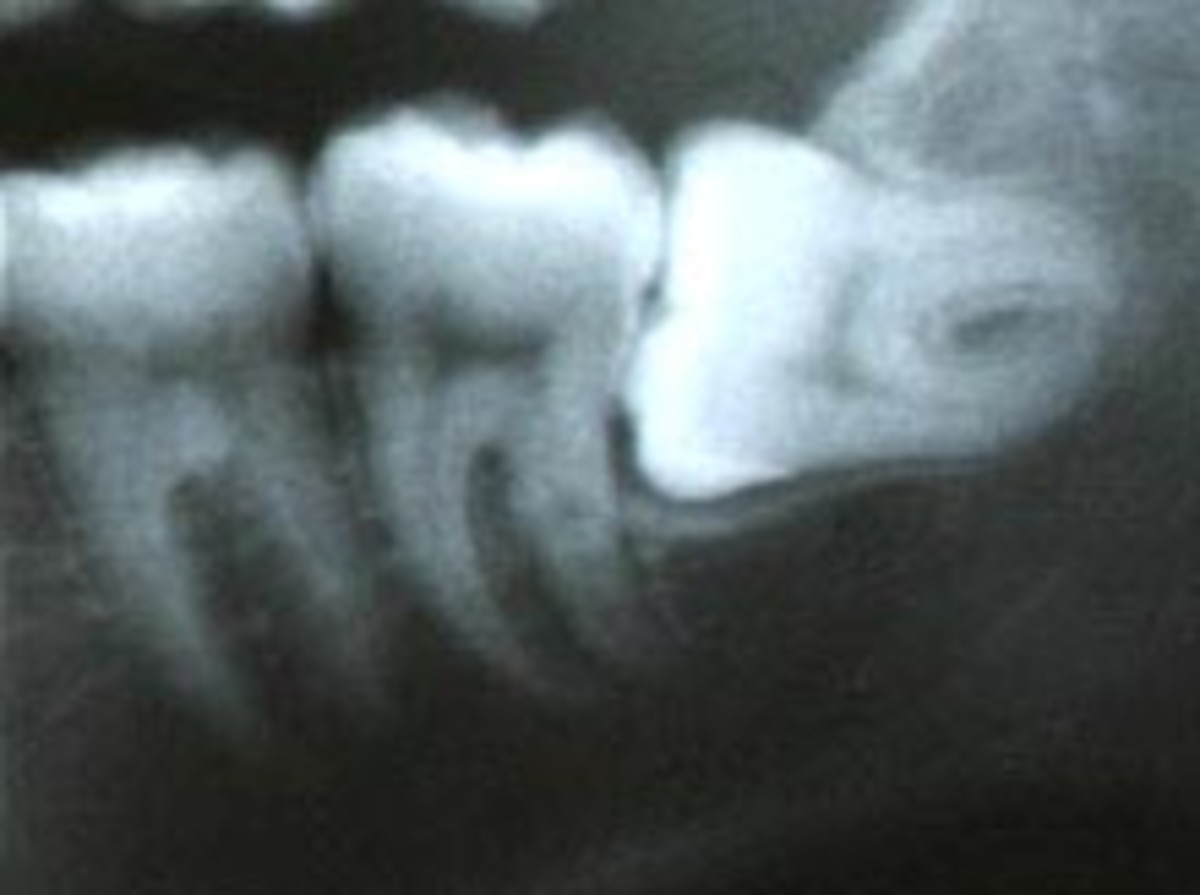
Each patient was selected on the basis of having 2 radiographic vertical defects with associated probing depths of >=5.0 mm following initial non-surgical therapy.
All patients completed a comprehensive medical and dental history exam. Only those defects with an intrabony component >=3.0 mm were included. Patients were then given an explanation of the study purpose including benefits and associated risks, as well as alternative treatment regimens. Finally, a signed informed consent was obtained (This study and consent forms were approved by the Institutional Review Board of the University Of Texas Health Science Center, San Antonio).
All patients received initial therapy including full mouth scaling and root planning with hand instruments and ultrasonic scalers, utilizing 2% xylocaine with epinephrine 1:100,000 for patient comfort when required. 2 visits were utilized to complete initial therapy with oral hygiene instructions and occlusal adjustment addressed when indicated.
Approximately 4–6 weeks following the hygiene phase of therapy, all patients underwent a re-evaluation examination to assess probing depth, attachment level, mobility, furcation involvement, and bleeding on probing.
All subjects were required to achieve at least an 80% modified O’Leary plaque index prior to progressing into the surgical phase of therapy.
Methods and materials:
Clinical measurements included both soft and hard tissue analyses. Soft tissue dimensions included:
- Probing pocket depth: free gingival margin to base of the pocket.
- Clinical attachment level: CEJ to base of the pocket.
- Recession: CEJ to free gingival margin.
Hard tissue measurements included:
- CEJ to alveolar crest.
- CEJ to base of the defect.
- Alveolar crest to base of the defect.
(The CEJ was utilized as a fixed point of reference if a restorative margin was not available for selection.)
Final inclusion into the study was based on intrabony defect depth, alveolar crest to base of the defect, intraoperatively.
Results:
Clinical evaluation of post grafting surgical healing revealed an excellent soft tissue response to both materials with no adverse complications neither seen nor reported.
Soft tissue findings revealed, after treatment, pocket depth was significantly decreased as compared to baseline values in both groups but the difference was not significant between the groups.
An evaluation of hard tissue findings at the six month re-entry revealed both treatment groups had improved significantly over baseline values with regard to bone fill, percent bone fill, and % defect resolution. Finally, crestal resorption was insignificant in each group with the DFDBA having 0.7 mm ±1.3 resorption and the BDX group demonstrating 0.8 mm ±1.2.
There were 4 sites which were non-responders, three being treated with DFDBA and one with BDX which have been eliminated.
Defects which were treated with the bovine derived xenograft, although not statistically different, did show an average of 1.0 mm more PD reduction, and 1.2 mm more AL gain. With regard to hard tissue results, the BDX treatment group exhibited an average of 9.0% greater bone fill and an average defect resolution 19% greater than that demonstrated by the DFDBA treatment group.
Strength and weakness:
- A post-hoc power of analysis revealed that approximately 86 patients would be required to demonstrate a significant difference between materials.
- The defects in this study were measured only in a 2-dimensional plane with a Loma Linda 20.0 mm probe to the deepest point of the defect.
- The BDX group appeared to exhibit a greater volumetric bone fill which could not be assessed by the 2-dimensional measurement technique utilized.
- Different methods of measurement have been utilized in other studies during baseline and re-entry procedures to evaluate graft performance.
- DFDBA was chosen as the positive control since DFDBA is frequently used as a bone replacement graft. Comparison to a non-graft control was not done.
- Autogenous bone was considered as the positive control, but the inclusion of subjects with sufficient intraoral donor sites to fill deep osseous defects who presented with multiple defects would have placed additional constraints upon the study.
- It is important to point out the differing handling characteristics of the two materials evaluated.
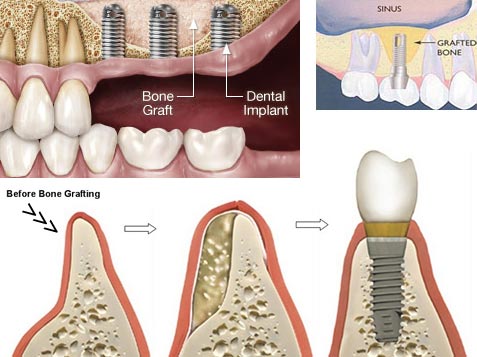
Bone Grafting in Dentisty