Experimental Hepatitis C Treatment
What is Hepatitis C?
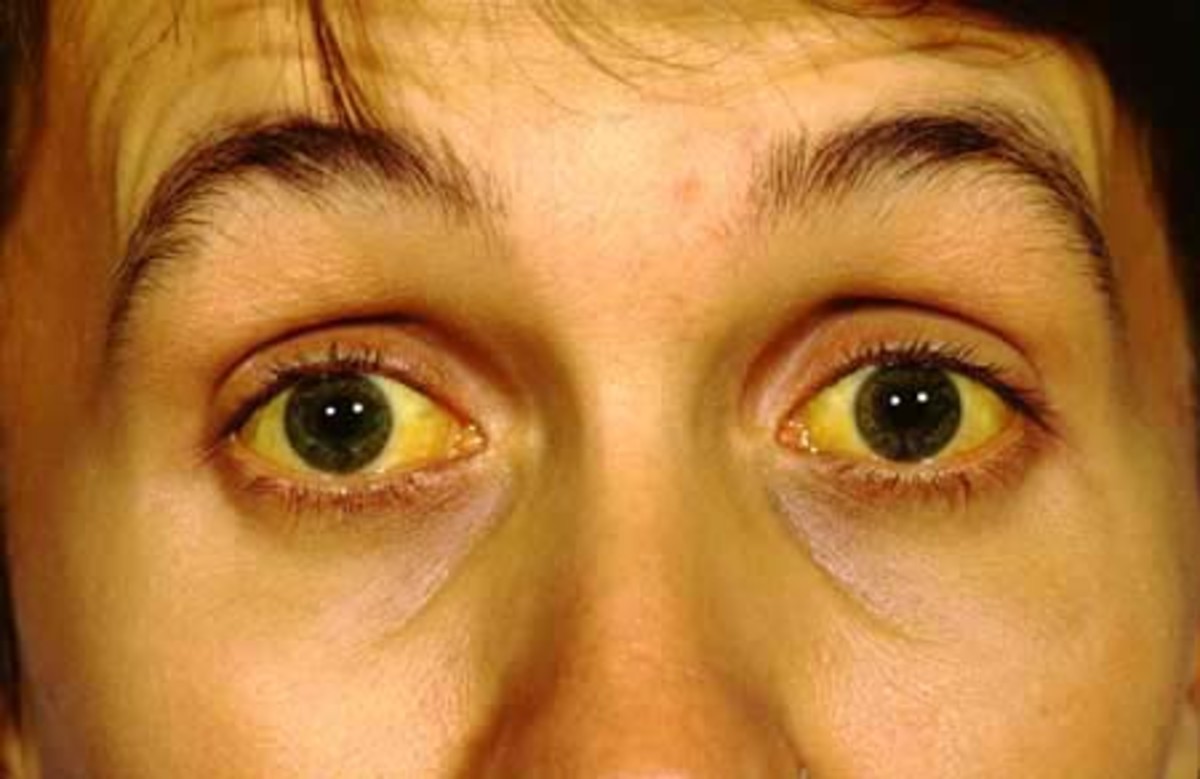
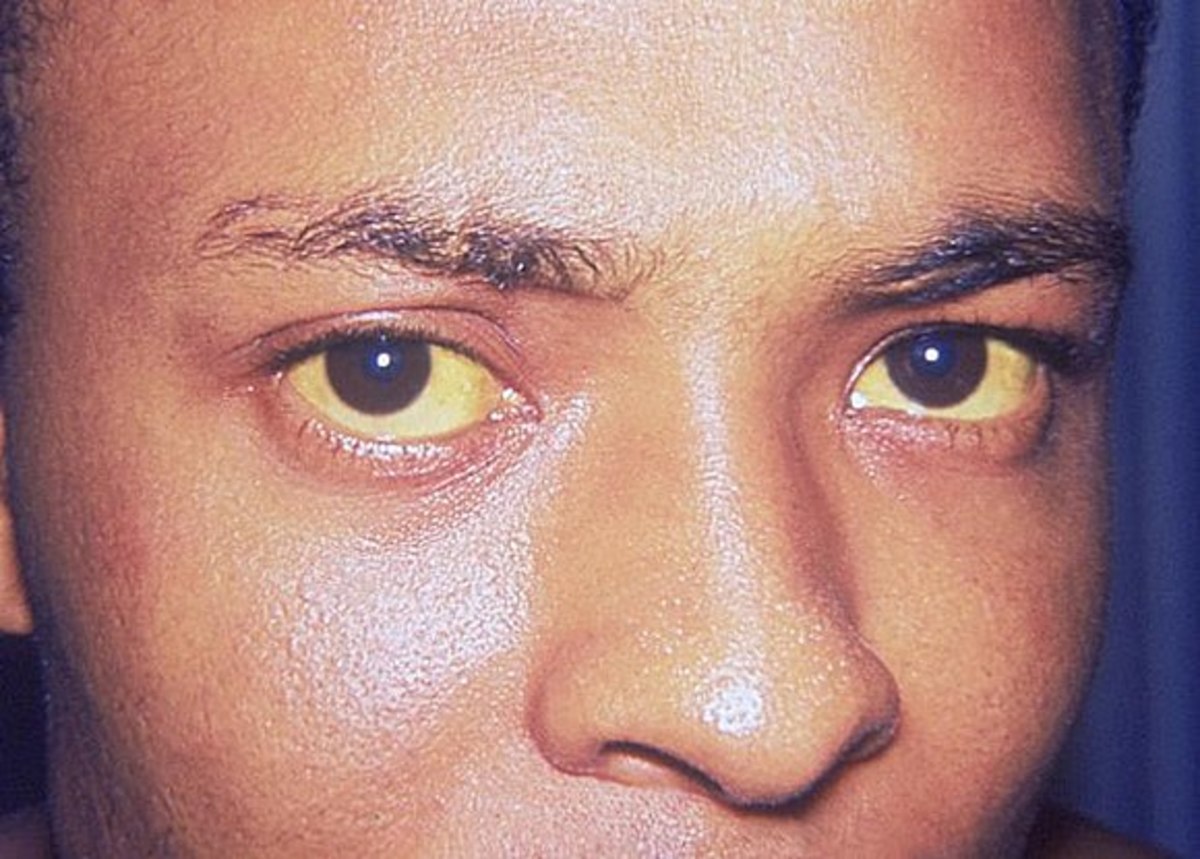
Hepatitis C is a viral disease affecting the liver. As a disease, the initial infection often goes unnoticed, but as the infection progresses it can cause liver fibrosis, and in advanced cases, scarring of the liver called cirrhosis. This cirrhosis stage is often apparent after many years of infection.
Those with cirrhosis have the potential to go on to develop liver failure or other complications. This includes liver cancer.
Despite it's potentially deadly nature the virus can only be transmitted through direct blood-to-blood contact. e.g. the virus is blood-borne and must involve contact of blood between the carrier and the uninfected.
Such viral blood-to-blood transfer typically involves intravenous drug use, but can also be transmitted in health-care workers who have inadvertently "stuck" themselves with contaminated "sharps" such as needles, scalpels, and sutures from infected patients.
An estimated 170 million people worldwide are thought to be infected. With three (3) to four (4) million of those infected being Americans.
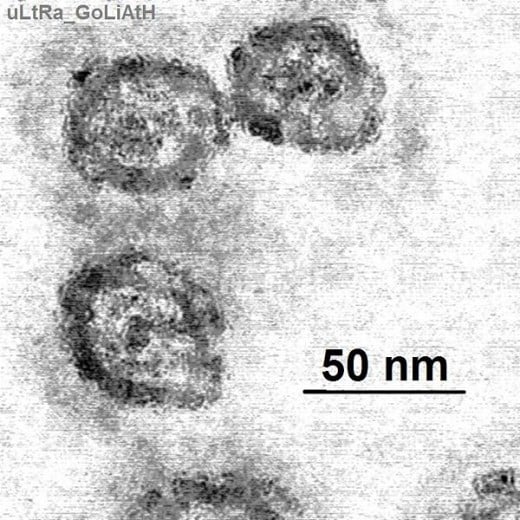
New Method Targets miRNA
miRNA is short for micro-RNA. A short segment of RNA incorporated into longer strands of this substance is called miRNA. By targeting a specific miRNA this new therapy has fewer side-effects, better short-term response, and best of all, a very low "rebound" rate.
As such the drug uses an entirely different method to fight a viral infection. To date the anti-virual has been tried on chimpanzees, the only other mammal capable of contracting the virus. It is currently undergoing human clinical trials.
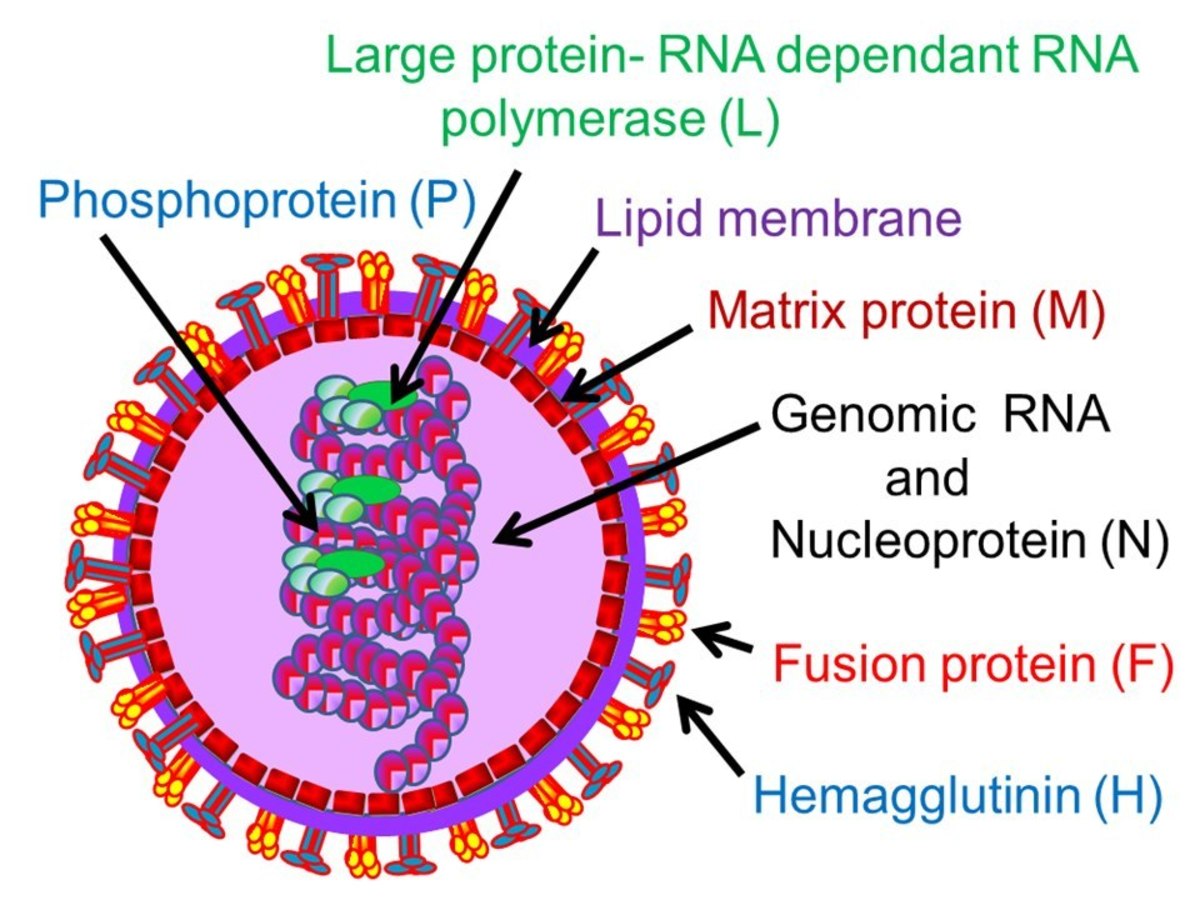
The new anti-viral is called an antisense drug. It works by binding to an miRNA segment present in the liver. As it turns out this segment is also used by the Hepatitis C virus to replicate.
The drug, called SPC3649, so far has shown no toxic side effects. It also does not allow development of resistance and appears to have lasting effects after treatment has stopped.
Current Therapy
Currently the treatment for Hepatitis C combines two antiviral drugs. Ribavirin and Interferon. Unfortunately this treatment has rough side-effects that often cause patients to stop using them. These side-effects include hair-loss, diminished energy levels, and nausea.
Worse, the drugs are only effective in about half of those going through the forty-eight week treatment. The reason for the diminished effectiveness is the viruses ability to develop a resistance to the treatment.
Considering the expense, time required, and mixed results this form of therapy leaves much to be desired.
SPC3649 Therapy
By contrast this new drug seems to be resistance proof. SPC3649 outwits the virus by bypassing the virus itself and targeting a short strand of RNA essential to the virus being able to replicate.
miRNA has been shown to play a very critical role in gene activity and replication by turning genes on and off. In 2005, Dr. Sarnow and his colleagues proved that Hepatitis C hijacks a particular piece of RNA called miR-122. This miRNA is typically found in liver cells. Hepatitis uses this miRNA to reproduce. To date, it is the only virus known to use this specific piece of miRNA.
Santaris Pharma A/S in Hoersholm, Denmark, and San Diego
developed a process to synthesize a short strand of DNA that binds very tightly to miR-122. This inactivates it. SPC3649, which contains this synthetic DNA, was injected intravenously into four
chimpanzees with chronic Hepatitis C infections for 12 weeks. At the end of the 12 weeks the chimps were observed for an additional 17 weeks. All animals displayed a drastic drop in viral activity with no indication of resistance.
Virus levels remained low through of the observation period. No strong viral rebound was observed either. The only noted side-effect of the treatment was a 45% drop in levels of
low-density lipoprotein. This is the so-called bad cholesterol. Cholesterol production is also a normal functions of miR-122. Of course, a reduction in bad cholesterol is a beneficial side-effect.
Santaris has begun safety trials in healthy volunteers. These volunteers also display a drop in cholesterol levels. This is clear indication that the drug is
working. Effectiveness trials are expected to begin next year. This means that the drug may be widely available in the next five to seven years.
Since the drug affects genes ability to turn on and off there is no clear understanding, as yet, as to how this drug will affect humans over the long term.
Disclaimer
The author owns not stock in Santaris Pharma nor was compensated in any way for this article. The author was not compensated with money, discounts or freebies for the contents of this article.