- HubPages»
- Health»
- Health Care, Drugs & Insurance»
- Prescription & Over-the-Counter Drugs
Isoniazid Overdose: Seizures And Other Life-Threatening Side Effects

Tuberculosis and Isoniazid
Tuberculosis (TB) is one of the most prevalent respiratory conditions that has plagued men since early times. It is caused by an organism of which, the most affects man is called Mycobacterium tuberculosis. It was one of the worst difficulties of the first-world countries several centuries ago; and has been ravaging many countries in Asia, Africa, and deep pockets of the world until now. Tuberculosis is very communicable, transmissible through unwary humans hence, the contagion cannot be easily halted. One of its most important characteristics is the presence of a unique cell wall which inhibits typical antibiotics to penetrate. Contrary to popular belief, tuberculosis can thrive not only in the lungs (or respiratory tract) but almost in any other organ of the body- not only decreases the likelihood of identification and let alone treatment. Now, enough of the foe that we can’t handle; as a contender for human beings, who was sent on the other side of the ring?
People have tried to develop medications that will counteract the said properties of the disease-causing agent aforementioned by specifically targeting the special characteristics of the organism above. Today, several combination drugs or fixed-dose therapy is employed. This means that there are several drugs added on one capsule to target the bacteria. The main prototypical drug that is used is isoniazid. Isoniazid (INH) or isonicotinic acid hydrazine is an antibiotic drug used to treat infection with Mycobacterium tuberculosis. It is considered as one of the first-line agents in the treatment of tuberculosis. Its mechanism of action of INH is inhibition of mycolic acid synthesis, which in turn, interferes with the cell wall synthesis. Basically, it blocks a certain component of in the production of proteins so the cell wall of the organism is not produced, hence destroying the bacteria- this complex blocks mycolic acid synthesis. This produces the bactericidal effect of INH. (Drew, 2013).
Isoniazid is well absorbed after oral administration. But, it may be delayed by intake of food and antacids. INH can also be given parenterally or through the veins. INH is widely distributed in the body (including the cerebrospinal fluid and breast milk), with a volume of distribution ranging from 0.6 to 1.2 L/kg. Protein binding is low (<10 percent). Metabolism of INH is via acetylation. Acetylation is genetically determined and varies among ethnic groups with Caucasians and African-Americans as slow acetylators and Asians as fast acetylators. The elimination half-life ranges from 1 to 1.8 hours in fast acetylators to 3 to 4 hours in slow acetylators. INH is eliminated mainly via the kidney. Slow acetylators excrete a higher proportion of unchanged drug. INH is also effective against atypical mycobacteria such as M. kansasii and M. xenopi. However, M. avium complex is resistant to INH (Drew, 2013).
Now, we know that we have a very rampant disease, opposed to a seemingly effective drug. They have been waging wars for centuries and we are yet to see the results. What’s in it for those who are taking the drugs? One thing we should worry about is a severe complication of over dosage of this drug. INH toxicity is often a complication of antituberculosis therapy. Acute INH toxicity often occurs as unintentional ingestion especially in children, suicidal intention or compensation for missed dosing schedule. Approximately 10-20% of adults receiving INH have elevated liver function test. A study of USPHS in 1978 stated that 10% of INH-treated patients with mild elevations of transaminase have hepatitis and liver failure (Weisgler et al., 2012). Susceptibility to INH-induced hepatitis and subsequent death increases with age. It is more common in females than in males.
Rome was not built overnight, and the same is true for the treatment for TB!
Some people argue that what's the likelihood that they get an overdose if they take a regimen of antibiotics for a week or two? Overdose may be seen since treatment for TB will be prolonged, not by a week, or a month, but a whopping 6 months at least! In many countries the basic regimen will last for 6 months, and may be prolonged dependent on the strain of the organism and its drug resistance. Overdoses are not farfetched.
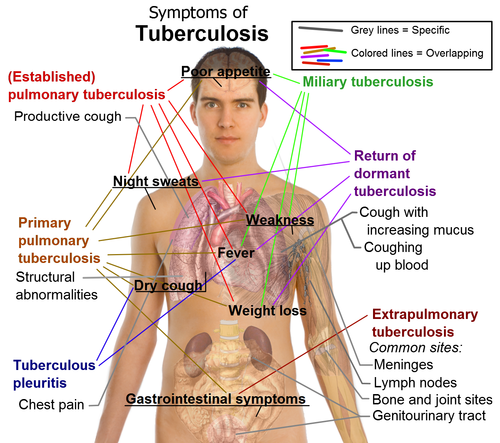
Signs and Symptoms of Isoniazid Toxicity
Acute overdoses of isoniazid have been identified with a classical triad of symptoms including seizures refractory to standard anticonvulsants, metabolic acidosis, and coma. If isoniazid is taken acutely, as little as 1.5 g (five 300-mg tablets) can cause toxicity (Desai & Agarwal, 2004). The toxic effects of isoniazid are usually manifested at higher dosages. Adverse effects include CNS peripheral neuropathy manifesting as paresthesia (feeling of numbness to tingling sensation) of the feet and hands, convulsions, and toxic encephalopathy. It was reported to be seen in 15% of patients taking upper limits of dosages of 10mg/kg body weight. In a study, it was found that there were no significant side effects when the drug was taken within the prescribed amounts. The most common adverse reaction was peripheral neuropathy which was present in people with other conditions such as diabetes, uremia, alcoholism, malnutrition, and HIV infection (Bhurgri, Rathore, Korijo, & Qazi, 2012).
Several cases of ingestion of massive amounts of isoniazid lead to acute isoniazid poisoning. It was estimated that 80mg/kg body weight produces severe central nervous system symptoms, and a dose of 125 mg/kg body weight is potentially lethal if not treated promptly (Khoharo, Ansari, Abro, & Qureshi, 2009). Acute isoniazid toxicity presents clinically 30 minutes–2 hours after ingestion. Its symptoms include vomiting, rash, fever ataxia, disarticulation, peripheral neuritis, vertigo , and stupor are common signs of poisoning, which are then followed by grand-mal seizures and coma. The seizures are particularly resistant to anticonvulsants and also to barbiturates which differentiate it from other forms of seizures (Cakmak, Atas, Soran, & Zeyrek, 2009).
For those interested, and is versed in the physiology and biochemistry of cells- here is the mechanism: Isoniazid binds to pyridoxal-5-phosphate, the active form of pyridoxine (Vitamin B). Pyridoxal 5-phosphate is a cofactor for enzymes involved in the gamma amino butyric acid (GABA) synthetic pathway (Kurzbaum, Katheeb, Maria, & Segalp, 2002). Acute ingestion of large quantities of isoniazid causes absolute pyridoxine toxicity since it acts on an essential cofactor of GABA. This decreases the threshold of inhibition from the central nervous system leading to seizures (Cakmak et al., 2009). INH also causes a decrease in GABA synthesis by inhibiting the pyridoxal phosphate-dependent enzyme glutamic acid decarboxylase. Therefore, seizures do not respond to conventional antiepileptic drug (Eyüboğlu & Derinöz, 2013). Isoniazid also inhibits lactate dehydrogenase, an enzyme that converts lactate to pyruvate in the liver resulting in lactic acidosis. Acute ingestion of as little as 1.5 gr. can produce toxicity; 6-10 gr. may be fatal if not correctly treated (Kurzbaum et al., 2002).

Pyridoxine is more commonly known as Vitamin B6! Although what is used in this case are not your usual tablets, but a more concentrated IV form!
Pyridoxine as an Antidote
A profound metabolic acidosis is usually present. This means that the whole body starts to become more acidic and if not controlled, will lead to more catastrophic sequelae. Whether this occurs before the onset of a seizure or thereafter is unclear. The exact mechanisms proposed involve the muscular activity from seizures, acidifying isoniazid metabolites and enhanced fatty acid metabolism (Judge, 2005). Whether this acidosis needs to be corrected with bicarbonate is controversial. Usually, pyridoxine alone corrects the metabolic acidosis (Kurzbaum et al., 2002). Parenteral pyridoxine administration is the main form of treatment for the uncontrollable convulsions. This also helps in recovering from the metabolic acidosis (Cakmak et al., 2009).
Pyridoxine also has a role in reversing coma. It is not only effective in treating INH-induced seizures, but also improves mental status due to a high dose of the drug. Higher doses than the ones used to control seizures may be necessary to improve consciousness (Sunumu, Coban, Dogan, Dirik, & Intoxication, 2013)
One of the identified complications of INH poisoning is rhabdomyolysis. Rhabdomyolysis is defined as damage to skeletal muscle resulting in the subsequent release of intracellular contents into the circulation, particularly myoglobin and CK (Eyüboğlu & Derinöz, 2013). Rhabdomyolysis can be a result of massive seizure activity and also a direct toxic effect of isoniazid or its metabolites on the muscle when ingested in massive amounts. The condition is detected with elevated levels of CPK and myoglobinuria (Blowey, Johnson, & Verjee, 1995).
Chronic forms of isoniazid toxicity are also presented in some individuals. The exact mechanism of isoniazid-induced liver toxicity is unknown, but it is widely accepted as idiosyncratic on some susceptible individuals. Recent studies suggest that hydrazine, and not isoniazid or acetylhydrazine, is most likely to be the cause of isoniazid-induced hepatotoxicity. It was noted to cause irreversible hepatic cellular damage (Tostmann et al., 2008).
Why should people be concerned?
Living in first world countries wherein tuberculosis is not common may signal that people not to care about this drug and its possible toxic side effect. However, we should note that the rest of the world has it and people carry the bacteria and may transmit it even when they are asymptomatic. That being said, the mere fact that you go to a confined public space, a subway, a mall, close contact with anyone (unknowingly afflicted) and you start to chuck in the meds, you need to be alarmed.
History of INH ingestion plays a key role in diagnosis. If 1.5 g (five 300-mg tablets) is ingested, it can cause toxicity. Doses larger than 30 mg per kg often produce seizures. Ingestion of the drug in amounts greater than 80 to 150 mg per kg can rapidly lead to death. If information regarding overdose is unavailable, family history of a recent positive skin test in any member of the household, including the patient, should be obtained. In acute INH toxicity, symptoms usually manifest within 30-45 minutes but may be delayed up to 2 hours when peak serum level occurs. Symptoms may include nausea, vomiting, diarrhea, irritability, lethargy, abdominal pain, confusion, dizziness and photosensitivity (Weisgler et al., 2012). Jaundice usually appears in more severe cases as a late manifestation. Physical findings usually resemble those of acute hepatitis. There is also RUQ tenderness, hepatomegaly, gingival bleeding or other manifestations of coagulopathy (Weisgler et al., 2012).
Laboratory Tests!
Serum INH levels are not correlated with severity of acute INH intoxication. Laboratory studies are often employed to determine complications of INH toxicity. Elevations of more than 3 fold of transaminases usually indicate INH toxicity and require discontinuation of the drug. High transferrin saturations associated with high ferritin levels suggest hemochromatosis, which often presents with transaminase abnormalities.
Blood gasses, serum electrolytes, glucose and BUN determination may be performed. Metabolic disorders are observed as lactic acidosis, hyperkalemia, hypocalcemia, hyperglycemia, and ketonuria. In cases of coma, ECG monitoring is recommended. ECG may also rule poisoning caused by drugs that affect QRS or QTc intervals.
Acute toxicity may present with leukocytosis and eosinophilia. The same CBC results may be observed in chronic toxicity with the addition of agranulocytosis, hemolysis, and anemia.
Imaging studies may be done, specifically, chest x-ray, to provide an evidence of tuberculosis and possible INH usage.
In summary, the main point is for the public to be aware that such conditions exist no matter how rare it may be. Once these symptoms are seen, it is highly advised and most prudent to seek medical care as soon as possible.
Treatment for Acute Isoniazid Overdose
In the medical healthcare facility, Acute overdose may be treated with three phases. First, is to control the problem here and now. The life threatening problem at hand is the priority! Then, the antidote and medication for rescue are given to counteract the metabolic problems. Lastly is the supportive care and long-term care to the road to recovery.
I. Control of life-threatening events
A secure airway is established and maintained if the patient is having seizures, is comatose or is unresponsive. Patients with a clinically severe INH overdose are at high risk for aspiration and prolonged hypoxia.
During any period of drug-induced paralysis, the pupillary response may help to determine the presence of an active seizure. Pupillary reactivity is not typically affected by nicotinic neuromuscular antagonists (eg, succinylcholine). Nonreactive pupils suggest active seizures and require therapy with pyridoxine as well as GABA agonist medications, such as benzodiazepines
Intravenous access should be obtained, and fluids should be administered. Diazepam (Valium), 5 to 10 mg administered intravenously, is the initial approach to seizure control, with the dose repeated as necessary. Diazepam has been found to be more effective in controlling isoniazid-induced seizures than anticonvulsants such as phenytoin (Dilantin) or barbiturates.
The acidosis associated with isoniazid toxicity appears to be lactic acidosis secondary to the seizure activity. Therefore, as the seizures are controlled, the acidosis usually decreases in severity. Since sodium bicarbonate may assist in correcting severe cases of acidosis, its administration should be considered if the pH is less than 7.1. A good starting dose is 1 to 3 mEq per kg, with frequent monitoring of blood gasses to guide further bicarbonate administration.
II. Correction of GABA deficiency by pyridoxine replacement
Pyridoxine (vitamin B6) binds to INH, repletes stores of pyridoxine and pyridoxal 5' phosphate and facilitates the production of GABA. It should be administered in a dose equivalent to the suspected maximum amount of isoniazid ingested (i.e., gram-per-gram replacement). If the amount of ingested isoniazid is unknown, 5 g of pyridoxine is given intravenously over five to 10 minutes. Repeat dosing may be needed for persistent seizure activity and may also be used to reverse deep coma. Pyridoxine is a water soluble vitamin with a wide therapeutic margin. Acute pyridoxine-induced neuropathy is reported at doses of 1 g/kg, a dose unlikely to be required for the management of INH overdose.
III. Supportive Care
After the initial stabilization efforts, attempts should be made to prevent the absorption of isoniazid and to hasten elimination of the drug. Gastric lavage is indicated if it can be done within one hour of isoniazid ingestion. Charcoal, if administered within one hour of the ingestion of isoniazid, has been shown to be effective in preventing absorption of the drug. Charcoal should initially be given as a slurry with sorbitol. The usual charcoal dose is 30 to 100 g for adults (1 to 2 g per kg) and 15 to 30 g for children (1 to 2 g per kg). The sorbitol dose in adults is 1 to 2 g per kg, with a maximum dose of 150 g. In children, the sorbitol dose is 1.0 to 1.5 g per kg to a maximum dose of 50 g.
If diazepam fails to control seizures or pyridoxine does not reverse coma, other interventions may be considered. Hemodialysis has been used to lower serum isoniazid levels. Thiopental has been used to treat refractory seizures that did not respond to 12 g of pyridoxine.
References
- Bhurgri, G. R., Rathore, M. I., Korijo, H. B., & Qazi, M. A. (2012). Toxicity of Isoniazid (INH); Role of biochemical monitoring tests and history of weakness. The management of pulmonary tuberculosis in placebo-controlled study. Professional Med J, 105, 105–111.
- Blowey, D. L., Johnson, D., & Verjee, Z. (1995). Isoniazid-associated rhabdomyolysis. The American journal of emergency medicine, 13(5), 543–4. doi:10.1016/0735-6757(95)90168-X
- Cakmak, A., Atas, A., Soran, M., & Zeyrek, D. (2009). Acute Isoniazid Poisoning Presenting With Convulsions And Coma. Harran Üniversitesi Tıp Fakültesi Dergisi, 6(1), 47–48.
- Desai, V. A., & Agarwal, S. B. (2004). Isoniazid Toxicity. Journal, Indian Academy of Clinical Medicine, 5(1), 83–85.
- Drew, R. 2013. Isoniazid: an overview. Retrieved from http://www.uptodate.com/contents/isoniazid-an-overview?source=see_link.
- Eyüboğlu, T., & Derinöz, O. (2013). Rhabdomyolysis due to Isoniazid Poisoning Resulting from the Use of Intramuscular Pyridoxine. The Turkish journal of pediatrics, 55(3), 328–30. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24217082
- Judge, B. S. (2005). Metabolic acidosis: differentiating the causes in the poisoned patient. The Medical clinics of North America, 89(6), 1107–24. doi:10.1016/j.mcna.2005.06.011
- Khoharo, H. K., Ansari, S., Abro, A., & Qureshi, F. (2009). Suicidal Isoniazid Poisoning. J Ayub Med Coll Abbottabad, 21(2), 178–179.
- Kurzbaum, A., Katheeb, M., Maria, G., & Segalp, A. (2002). Acute lsoniazid poisoning. Israeli Journal of Trauma, Intensive Care and Emergency Medicine, 2–4.
- Okutur, S. K., Borlu, F., Ersoy, C. Y., & Paksoy, F. (2006). Acute Isoniazid Intoxication : Convulsion, Rhabdomyolysis, and Metabolic Acidosis. Turk J Med Sci, 36(6), 397–399.
- Rao, R.B. 2013. Isoniazid (INH) Poisoning. Retrieved 26 December 2013 from http://www.uptodate.com/contents/isoniazid-inh-poisoning#H10.
- Romero, J.A. and F.J. Kuczler. 1998. Isoniazid Overdose: Recognition and Management. Retrieved 26 December 2013 from http://www.aafp.org/afp/1998/0215/p749.html.
- Sunumu, O., Coban, E. K., Dogan, V. B., Dirik, A., & Intoxication, I. (2013). A Cause of Status Epilepticus in Turkey: Isoniazid Intoxication. The Journal of Psychiatry and Neurological Sciences, 26(4), 395–397. doi:10.5350/DAJPN2013260411
- Tostmann, A., Boeree, M. J., Aarnoutse, R. E., de Lange, W. C. M., van der Ven, A. J. An M., & Dekhuijzen, R. (2008). Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. Journal of gastroenterology and hepatology, 23(2), 192–202. doi:10.1111/j.1440-1746.2007.05207.x
- Vianzon, R. & Garfin, A. 2011. The tuberculosis profile of the Philippines, 2003-2011. Western Pacific Surveillance and Response Journal, 4(2).
- Weisgler, RA. 2012. Isoniazid Toxicity Treatment & Management. Retrieved 26 December 2013 from http://emedicine.medscape.com/article/180554-treatment#a1156.
- Wills, B. (n.d.). A 23 Year Old Woman who Presents with New Onset SE.
© 2016 LM Gutierrez