- HubPages»
- Health»
- Health Care, Drugs & Insurance»
- Prescription & Over-the-Counter Drugs
MMR vaccine – side effects and what to do
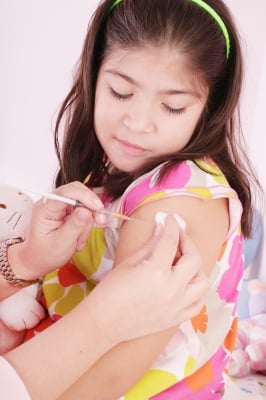
In the UK, the first dose of MMR vaccine is given at 13 months of age and the second dose is given around pre-school time (between 3 years and 4 months old and 5 years old).
I have heard cautions from mothers about giving this vaccine to their children; nevertheless I never found a good reason not to vaccinate my children. I have a 3 and a half years old and a fourteen months old, so both were due a course of vaccine very recently. So I did some research as I wanted to know the reality behind this.
What does MMR stand for?
MMR stands for Measles, Mumps and Rubella (German measles). These are very contagious viral diseases that affect mostly children. They are transmitted airborne.
Measles, Mumps and Rubella diseases
Measles
| Mumps
| Rubella
| |
|---|---|---|---|
Initial symptoms
| Fever, runny nose, bloodshot eyes, and tiny white spots on the inside of the mouth
| Headache, muscle pain, low-grade fever
| Mild fever, nausea and a temporary rash
|
Following symptoms
| Rash starts on the face and upper neck and gradually spreads to the rest of the body
| Swelling of one or both parotid glands
| |
Symptoms appearance
| 10-12 days
| 2-3 weeks
| 2-3 weeks
|
MMR vaccine
A vaccine is a preparation that gives immunity to a specific disease. This preparation has to be inoculated to provide immunity. Vaccines do not provide a hundred per cent immunity in every person; however, it is essential to receive all the doses of a specific vaccine to eliminate the possibility of becoming infected with a disease.
MMR vaccine is a “live” or attenuated vaccine, this means that they contain live microorganisms which either have been disabled their viral property or it is reduced, therefore the vaccine can activate the immunological response in the individual. Two doses of the vaccine protect 99.7% of the immunized individuals according to the National Network for Immunization Information.
MMR vaccine brand names
My research found the following brand names that are used in the United States and in the United Kingdom:
Vaccine
| MMR II
| MMR Vax pro
| PRIORIX
|
|---|---|---|---|
Company
| Merck Sharpe, Dohme (subsidiary of Merck)
| Sanofi Pasteur
| GlaxoSmithKline
|
Country
| US
| UK
| UK
|
Composition
| |||
Measles Virus Vaccine Live
| Enders' Edmonston strain produced in chick embryo cells
| Enders' Edmonston strain produced in chick embryo cells
| Schwarz produced in chick embryo cells
|
Mumps Virus Vaccine Live
| Jeryl Lynn (B level) strain produced in chick embryo cells
| Jeryl Lynn (B level) strain produced in chick embryo cells
| RIT 4385 produced in chick embryo cells
|
Rubella Virus Vaccine Live
| Wistar RA 27/3 strain produced in WI-38 human diploid lung fibroblasts
| Wistar RA 27/3 strain produced in WI-38 human diploid lung fibroblasts
| Wistar RA 27/3 strains produced in human diploid (MRC-5) cells
|
MMR and autism controversy
MMR vaccine controversy
In February 1998, The Lancet published an article associating the development of autism with MMR immunization. This publication triggered some panic among parents since it was broadly covered in the media. After more research was done, no evidence between the MMR vaccine and autism was proven. Therefore, in 2004, The Lancet fully retracted.
Several serious outbreaks of measles and mumps have happened worldwide, this has costs thousands of dollars per case.
Unfortunately, it seems that the media covered the connection between MMR vaccine and autism more extensively than the retraction, so still parents are concerned about the vaccine adverse events.
Who should wait to get the MMR vaccine?
People who are sick at the time scheduled for the vaccine should wait until they are not sick any more. Pregnant women should wait until after birth. If a woman gets the vaccine, she should wait at least 4 weeks to conceive.
An important caution is to avoid receiving another “live” vaccine within 4 weeks of receiving MMR vaccine.
Who should not get MMR vaccine?
People who have had a life threatening allergic reaction to the antibiotic neomycin or to any of the components of the vaccine or to a previous dose of the MMR or MMRV vaccine should not get the MMR vaccine.
MMR vaccine side effects
Every single pharmaceutical product has side effects. Vaccines are no exception. However, the disease is far worse than the side effects. Measles, mumps and rubella have no specific treatment; therefore, it is crucial to receive immunization against it even though side effects can occur.
Priorix side effects:
More common side effects include redness at the injection site, fever 38°C (rectal) or 37.5°C (axillary/oral). Common side effects are rash, pain and swelling at the injection site, fever >39.5°C (rectal) or >39°C (axillary/oral) and upper respiratory tract infection.
Uncommon side effects include otitis media, lymphadenopathy, anorexia, nervousness, abnormal crying, insomnia, conjunctivitis, bronchitis, cough, parotid gland enlargement, diarrhea, vomiting
Rare effects are febrile convulsions and allergic reactions.
MMR II side effects:
The most common side effects are fever and temporary joint pain (mostly in teenage or adult women). If a rash occurs it is usually mild. Rare side effects include swelling of glands in the cheeks or neck. Very rare side effects are seizure caused by fever and temporary low platelet count. Less than 1 out of a million doses can cause a serious allergic reaction.
Several other severe problems have been reported after a child gets MMR vaccine, including: Deafness; long-term seizures, coma, or lowered consciousness; permanent brain damage. These are so rare that it is hard to tell whether they are caused by the vaccine.
Source: CDC (Centers for Disease Control and Prevention) MMR Vaccine Information Statement April 2012.
Would you give the MMR vaccine to your children?
What to do if you or your child has a moderate or severe side effect?
First of all, call your doctor. In the UK call either your GP or NHS Direct. Do not jump to conclusions or attempt to self-medicate. Give the doctor details about the date and time when the vaccine was given and when the reaction started.
Second, fill out an adverse event form:
- In the US it is called Vaccine Adverse Event Reporting System (VAERS) form co-sponsored by the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA).
- In the UK it is called Yellow Card and it is controlled by the Medicines and Healthcare products Regulatory Agency (MHRA).
Filing these forms helps government to assess the safety of medicines and vaccines in the market to ensure public safety.