Radioactivity of Tobacco Smoke from Cigarettes


Tobacco's radioactivity was widely reported in 1964. News reports were that tobacco contained significant radioactivity, the most noteworthy of which were the radioisotopes, 210-lead and 210-lead decay products, most notably, 210-polonium. To some people, scientists and tobacco industry workers, this may not have been new information. To most of those who smoked tobacco, it came as a big surprise and a somewhat scary revelation.
Back then, it was heard, "Who knew ?"
After all, it had been not quite ten years since the dropping of atomic bombs and the resulting release of huge quantities of radioactivity, killing thousands of people with only several bombs. Thus, the mention of radioactivity from tobacco smoke should have been enough to frighten tobacco smokers and those subjected to the smoke from their smoking.
What you don't believe will not frighten you very much, if at all
The fact is, however, that the news back in 1945 and the news in 1964 did not register with all that many people. Smokers continued smoking tobacco. Did the news now being spread to the whole population about radioactivity in tobacco serve to make tobacco smokers even more nervous about their smoking habit ? Tobacco smoking seemed to increase rather than to decrease. Today, years later, people still smoke tobacco as though their lives depend on it. Indeed their lives do depend on it, rather, the shortening of the lives of people who inhale tobacco smoke depend on tobacco smoking.
I will do what I can to make a believer of you
Without reciting a lot of technical information or using laboratory jargon to explain how radioactive substances came to be in smoking tobacco, I will set out to explain the situation as best I can for the benefit of "non-jargoners" and those who prefer simpler images more so than masterpieces,
"Everywhere" is a big place
Almost everywhere you might travel on this earth, a small amount of radioactive elements will be present in the soil beneath your feet. Ordinarily, these "hot" substances stay put. So if there is some radioactive uranium, thorium, radium, or others hanging around underground, there they will stay unless someone displaces them somehow. Nothing much to worry about, right?
Maybe if I "bug" you about the deal
Ever since I was a youngster I have been fascinated by the way caterpillar bugs stayed around for a time and then, suddenly, they turned into butterflies or moths. Then I got older and learned that radioactive atoms do much the same thing. Now they are "this" and, all of a sudden, they become "that."
The problem with these radioactive elements is to be found in the "active" part of that word. They all do the sort of thing that caterpillars do. They stop being like caterpillars and become like moths or butterflies. Then they change colors, size, or voice and become something else. Eventually they quit being "active" and, in their old age, they stop their nonsense and sit around like some sort of a quiet lump of whatever kind of something they have come to be.
You might ask, "Why should that be a problem?" The fact is that it is not always a problem that radioactive elements in the ground start off as one thing and become other things. When they quit doing the caterpillar-butterfly thing they are finally into permanent and unchanging quiescence.
Change may not always be for the best
It is what they do whenever they change form along the way that they can be a problem to who or what is close by.
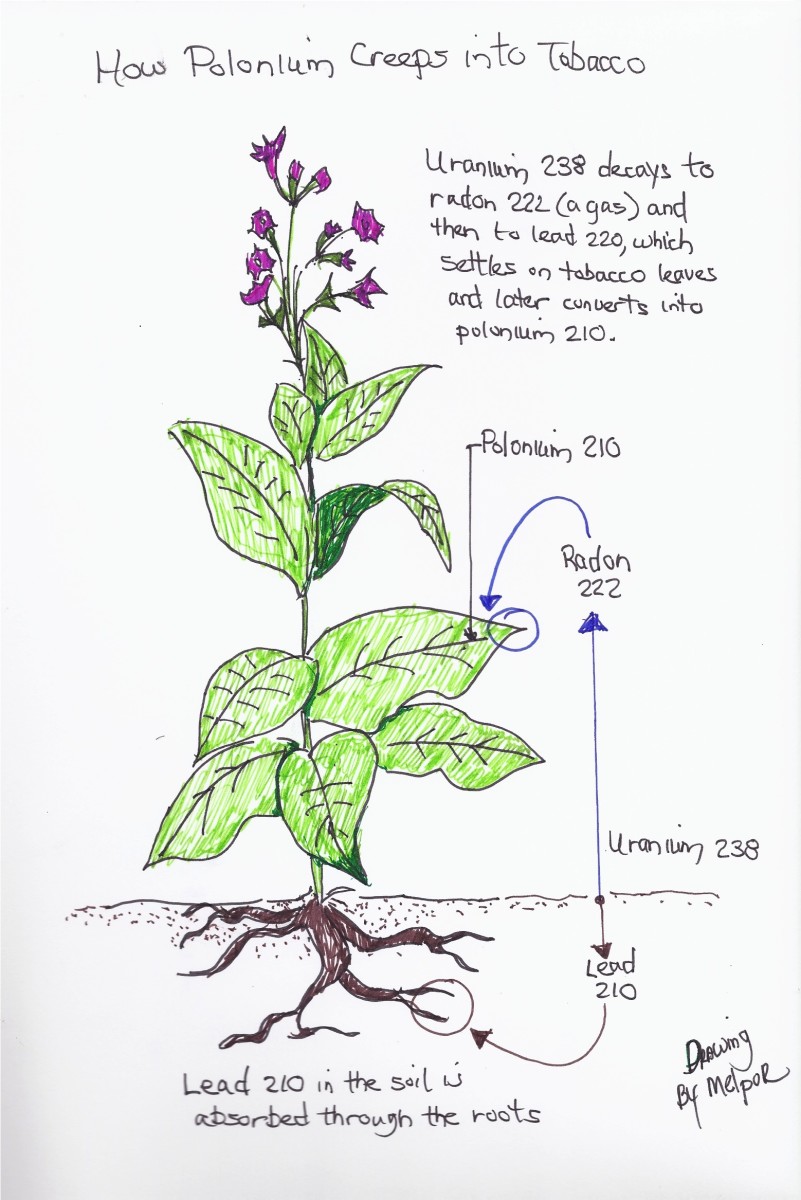
As for the uranium-thorium-radium kinds of "caterpillars," the critter with the wings that comes out of the always-changing chemical is a radioactive element. Sooner or later it is a gas that likes to fly away from its initial dirt home into the air above. That gas is known by several different names, radon gas or 222-radium. Radon is radioactive and exhibits its radioactivity by an eventual spitting out of an alpha particle (similar to the nucleus of a helium atom). This initiates the "birth" of progeny atoms (often called daughter products) that keep the family tradition of radioactive decay going. The almost ultimate descendant atom is 210-polonium. About half of any collection of radon gas will start its birthing activity every 3.8 days (its half life; that is, half of its atoms become something else in close to four days and half again every almost four days again and again until the collection's last atom goes ).
Sometimes the youngest of the bunch is the worst of them
The last kid into that radon nursery is the atom of 210-lead. Half of those little lead atoms decide that they, too, are radioactive and need to pop every 22.3 years. (That sort of time is remindful of a human's wait until young adulthood.)
Whenever an atom of 210-lead pops, it kicks out an electron from deep inside and, for a very brief time (half life time of 5 days), becomes an atom of 210-bismuth. Whenever the 210-bismuth gets ready, it, too, throws out one of those beta particles (an electron from its nucleus) and, ipso facto, there appears an atom of 210-polonium in its place. Not nearly as sleepy as was its "mama," that 210-lead, this newcomer, the 210-polonium has a 138-day half life. When this polonium atom goes away which it eventually will do, the last of the family remaining will be a little atom of 206-lead, the non-radioactive kind of lead metal, in its place.
Radon's "grandchild" inherited some nasty genes
Polonium's send off is signaled by its blasting out of one of those heavy (4 atomic mass units) alpha particles much like fireworks on the Fourth of July. By the time each alpha particle quits its flying and gets a chance to look back, its parent, 210-polonium has been "alpha-chemed" into an atom of sound asleep, non-radioactive (stable) 206-lead.
What all of this family radioactivity tradition means, once you boil it all down, is that the radon gas is responsible for the birthing of some "hot lead" and some "hot" polonium - a really "cool" deal, once you consider it.
It is quite a circus this radioactivity puts on. But, what in the world does all of that "hot" chemical stuff have to do with tobacco smoking and with the tobacco leaves that people burn so that they can inhale tobacco smoke?
Now, let's talk some about tobacco plants and how they grab up this radioactivity
As do other plants, tobacco plants take in water-dissolved chemicals through their roots and those are distributed throughout. Why is it that tobacco leaves are so loaded down with radioactive lead and its progeny?
So, let's talk some about tobacco plants - in particular, the tobacco leaves that people put afire so as to have tobacco smoke to inhale.
If you look at a field of growing tobacco plants, you will notice that the tobacco leaves are very broad and very long. The leaves overhang the growing soil like big, green umbrellas. Up and down the rows of tobacco plants, the leafy umbrellas are trapping the air below them like some sort of organic roof. On the undersides of these tobacco leaves are lots and lots of sticky little hairs (called trichomes).
One thing about these tobacco fields that you are not going to notice are the tiny atoms of 210-lead floating around in the air trapped beneath the tobacco leaves. The amount of that radioactive lead depends on the amount of radon that floated up from the soil below - the more uranium, thorium and radium in the soil, the more radon gas (222-radium) there will be in the air above it. Seems reasonable enough to me.
Paradoxically, to grow more and better tobacco, tobacco farmers who can afford it (and most can) dose their tobacco-growing fields with lots of fertilizer, especially the kind of fertilizer that contains a high percentage of phosphates. The preferred fertilizers typically contain phosphates derived from crushed and treated apatite mineral. The problem with this is that apatite is loaded with uranium, thorium, and radium. It is difficult to think of a better way to have tobacco field air more loaded with radon gas than to load its soil down with uranium, thorium, and radium.
As you understand from the complicated family history of radon gas and of typical tobacco farming, there will be some radon atoms in the air underneath all of those tobacco leaves. That means that there will be lots of different kinds of radioactive atoms being produced and floating around there, too. With more radon, there will be more "other stuff," all of it in the same family and all of it thinking about becoming radioactive lead and radioactive polonium. If the soil in which the tobacco is growing is emitting lots of radon gas, there will be lots of "hot" stuff floating around in the air under the tobacco leaves.
This "hot" stuff is made up of atoms that all possess electrical charges. Much like the grit that collects on electrostatically charged computer monitors and television screens, the radioactive decay products of floating radon are attracted to dust particles (aerosols) that are floating around with them. Those sticky hairs (trichomes) on the undersides of the tobacco leaves pick up the aerosol-radioactive atom combinations when the pairs bump into them.
Once stuck onto the trichomes, the aerosol-radioactive atom pairs stay stuck. Not only do they remain on the sticky trichomes out there in the tobacco fields, they remain in place all throughout the tobacco leaf harvesting, the long duration of the curing of the leaf, and the production of the marketable tobacco, and its packaging for the consumer.
There is nothing quite like inhaling hair smoke
When the tobacco smoker sets fire to his or her tobacco so as to inhale its smoke, or when the tobacco leaf product is otherwise sniffed, snorted, chewed, or swallowed, the smoke and vapors of the hairs or the little trichomes themselves are taken inside the body - and their radioactive passengers travel right along with them.
That fairly well summarizes the trail of the radioactivity from the dirt and fertilizer beneath the tobacco plants to the burning of the tobacco and inhalation of tobacco smoke by the smoker and by those around the smoker who are exposed to the smoke.
"Yes, but what is so bad about the radioactive lead on the tobacco leaf?"
It is not the truck that carries the bomb, it is the bomb carried by the truck
The answer might well be, "By itself, there is probably not much about the 210-lead over which to worry . It is what the 210-lead turns into that is really bad. In addition to that, the "hot" lead atoms that a person inhales from tobacco smoke get plastered to the sensitive linings of the lungs and to all of the breathing tubes that the tobacco smoke goes through to get in and out of the lungs. If any of that 210-lead gets into the blood stream, it goes here and there in the body.
"And - beyond those bad things, 210-lead eventually decays to become 210-polonium. Radioactive polonium is hell on wheels bad. even a teeny-tiny bit of 210-polonium is really bad news once it gets inside of you."
Think about that for a moment or more.
Twenty-two-plus years is a long time, and another twenty-two plus years on top of that make it even longer, ad infinitum
210-lead has a half life of almost 22.5 years. When it decays, out pops a beta particle - an electron. Beta particles do not do you any good when they hit something inside of you, but they usually are not too destructive to your body parts unless there are a lot of them coming at you constantly like a swarm of bloodthirsty mosquitoes. It is the 210-polonium that comes along following that 210-lead that is the bad stuff about which to worry.
Stuck with tarry glue from tobacco smoke
There sits the 210-lead, unfortunately stuck onto some of the sensitive lining, the epithelium of the breathing tubes and also of those important little sacks in the lungs, the alveoli, wherein the important exchanges of oxygen and waste gas of the body take place.
Those are the places where most of the really dangerous 210-polonium is born. If a person were to take in (smoke) tobacco only once during their entire lifetime, the probability of being harmfully zapped by 210- polonium produced from 210-lead deposition would be not much.
Again and again, over and over, more and more
However, when people smoke tobacco, they don't smoke it once in a lifetime. They do so again and again, day following day, year following year. The habit-forming nicotine from the tobacco keeps them returning for some more tobacco smoke, time and again.
210-lead deposition continues over time. The more 210-lead deposited on sensitive body tissues, the more 210-polonium is produced on the spot. Most of those deposit sites are probably in breathing-system-related locations, but both the radioactive lead and its progeny are not restricted to breathing locations. They can move along to many other locations, and so they do. But, wherever the 210-lead goes, it eventually gives way to its decay product, 210-polonium.
And - that is where real trouble can start.
The bomb is exploded where it hurts the most
210-polonium (which owes its life to that 210-lead) spits out a nasty alpha particle when it does what its radioactive forebears did. It decays. Instead of emitting a little beta particle as did its very short-lived parent, bismuth, and its grandparent, lead, the 210-polonium lets loose an energetic (5,3 million electron volt) alpha particle. When that alpha particle slams into sensitive, growing body tissue, it produces a thick trail of ionization; that is, tissue destruction and mutative genetic changes occur. (Note: an alpha particle in air typically will produce approximately 40,000 ion pairs per centimeter of particle travel - pieces of molecules each with an opposite electric charge.)
Sometimes you fight back, and sometimes you cannot
If the body's immune system is optimally functional and wide awake, some damage might be timely repaired and may be made well again. However, if there is some untoward genetic damage or tissue damage and a lapse in immune system protection, bad things can be brought about by collisions of 210-polonium alpha particles and molecules inside of affected body cells
A smoker has the really bad habit of continuously loading their breathing apparatus with bombs
The more tobacco smoke intake, the more 210-lead intake. The more 210-lead intake, the more 210-polonium given birth. The more 210-polonium, the more 5.3 million electron volt alpha particles. The more alpha particles, the more ionization. The more ionization, the more genetic damage within tissue cells. The more genetic damage within tissue cells, the more disease and harmful mutations in the person inhaling the tobacco smoke - intentionally or by breathing smoke-laden air.
It is all very logical. It is all correctable.
There must be something good to be said about not taking tobacco into ones body.
My 1964 tobacco smoke experiment
Here is some information from an actual tobacco smoking experiment I performed back in 1964 while working in the radioisotope laboratory of the Clinical Sciences Division of the USAF School of Aerospace Medicine. The experiment was not an official thing, but it came about due to the then fresh news report that "some scientists" had found that tobacco smoke contained a significant amount of radioactivity.
I was then a heavy smoker of tobacco. Neither I nor my bosses believed that report. I had all of the equipment needed to prove or disprove what we had read in that news report, and we had a large number of young and seemingly healthy tobacco smokers who happily volunteered to be tobacco smokers in my proposed tobacco smoke radioactivity experiment.
Here is how the experiment was set up and performed.
The tobacco to be smoked was in the form of filtered cigarettes, of one commonly available brand that I purchased all at one time from the base exchange (retail store).
One dozen volunteers, all of whom were tobacco smokers, would be closely monitored during the times of their participation so as to retain the consistency of smoking behavior (inhalation and exhalation times, portion of tobacco cigarettes smoked, and the like).
(1) Ten puffs of tobacco smoke not inhaled were sampled
(2) Ten puffs of tobacco smoke were inhaled and then exhaled for sampling
(3) "Washing" of residual tobacco from the last 25% of the smoked cigarette was sampled
The results of testing were expressed as net radiation counts per minute of the average from all of the results from the entire group of tobacco smoking volunteers.
Smoke not inhaled - 841 net counts per minute
Smoke after inhaling - 1 net count per minute
(Residual) 25% of unburnt tobacco - 1297 net counts per minute
You can figure out where those radioactivity counts went - the ones that stayed inside
There was a lot more of this testing that could be described, however, the above information is probably sufficient to paint the picture about where the radioactivity of tobacco goes when a person smokes it.
There were plenty of doubters as to the results from this experiment at that time (1964). With some good help from an expert typist and after consultation with some of my physician buddies (all of whom performed multi-week-long physical and laboratory testing of the then astronaut candidates), I wrote up this experiment and sent it to one of the medical journals for review and possible publication.
The journal folks laughed
The publication committee, so I was told, thought that this paper represented one of the more humorous of papers to come before them. It was not publication worthy, so I was told, after the editor confided to me that she had been told by the publication committee that they felt that this was one of the funniest of papers to ever hit their desk.
Thirty years later, nobody was laughing
Some years later (roughly 30 years) I had allowed myself to remain insulted by those folks such that, over those years, I continually collected all of the journal information I could find about the subject of radioactivity in tobacco. I was fortunate to work in one aspect of radiologic technology that allowed me to spend several hours a week in the journal stacks of a major university. I guess I read just about every journal that contained any sort of tobacco article. Mostly it was fun and I began to think about putting a big review paper together for sending to the "laughing journal."
When I felt that I had collected sufficient information about radioactivity in tobacco smoke, I put everything I felt to be of relevance onto paper. Then I asked the ex-president of the most famous and largest cancer hospital in the United States to review the paper. He thought that what I had to say had some merit and, "Gus, there is a bunch of stuff in your paper that is really new to me. Good work." He made some suggestions to me, one of which amounted to "Go back into your paper and get the chips off of your shoulders before you send it in to the journal." I did as he suggested and then it was shipped off to the same major radiological journal that had laughed at things 30 years earlier.
They did not have any problems with the paper's contents, and published it without any slicing. Since that time this review article has been cited by many who reported on their own tobacco smoke experimentation all over the world. That was enough to amaze me. There were some citations to the 1964 review paper in languages I could not read - Russian, Italian, and some written in languages that I did not recognize at all. I listened for laughing, but heard none.
It is really interesting that journals can both teach things and learn things over time. What is even more interesting is that people still smoke tobacco. I can understand how journals can digest information over time and welcome facts when they finally get to see and understand them. What I cannot understand is that people allow themselves to be addicted to tobacco smoking (like I used to be) and that they don't do what they have to do to win the war with their addiction.
The smoking habit seems to be always with me even though I quit smoking more than 50 years ago
So, for all of those who are going to allow tobacco smoking to sicken and kill themselves earlier in life than need be, I must sympathize with you in the form of this confession - "When I sit down for a nice cup of coffee of a morning, there are times when I find myself beating on my shirt pocket, searching for the pack of cigarettes that has not been in that pocket for the last 50 years."
Nicotine is one hell of an adversary, and that, dear friends, is a pure fact.