Temozolomide: A General Review
Overview
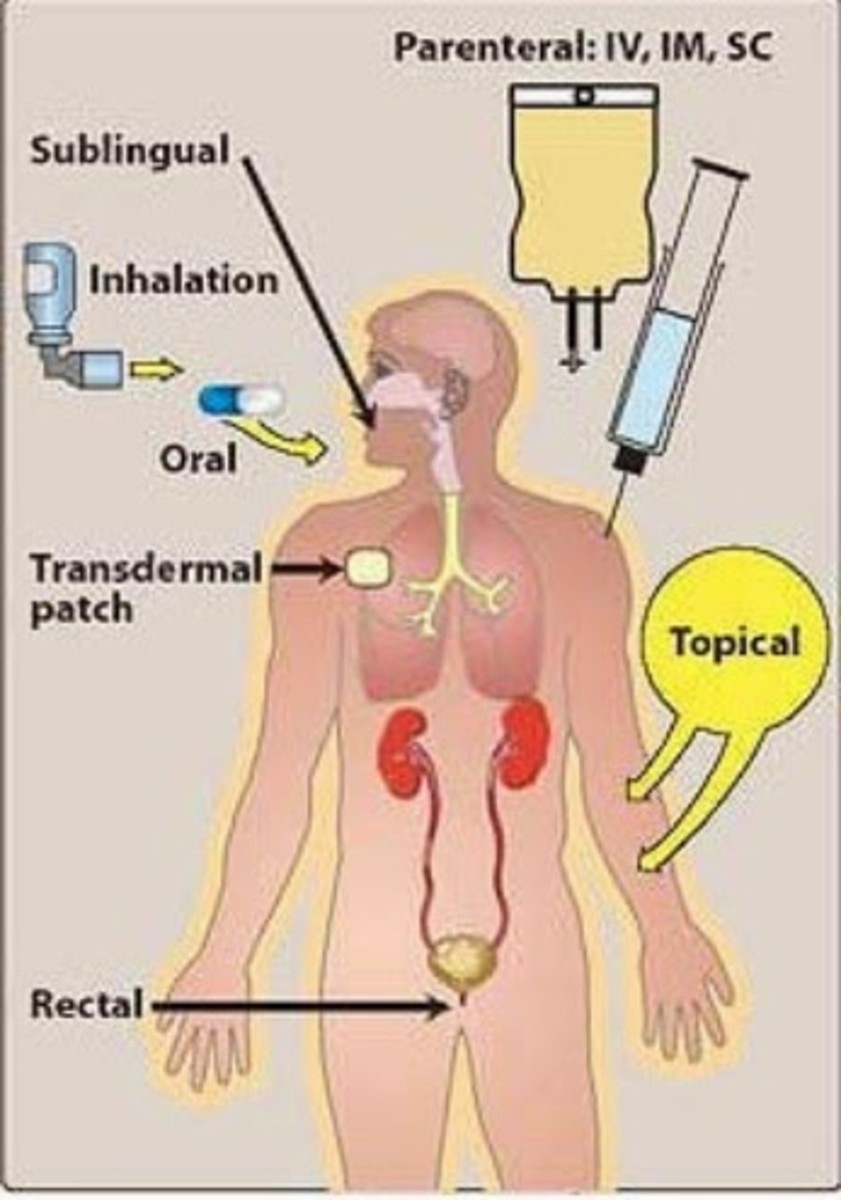
Temozolomide is an oral prescription cancer drug indicated for the treatment of adult patients with newly diagnosed glioblastoma multiforme and refractory anaplastic astrocytoma, a form of brain cancer. Many of the available chemotherapeutic agents come in injectable formulation. However, there are other cancer drugs that comes in oral formulations such as tamoxifen, iressa, estramustine, etoposide as well as temozolomide. With respect to the purpose of the article, Temodar, another name for temozolomide, is also available in injectable formulations. This is a very beneficial advantage because patients who were discharged from hospitals can actually be issued an oral formulation. Hence, self administration can be done.
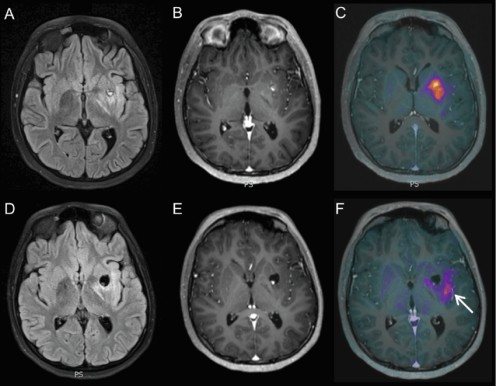
Anaplastic Astrocytoma, a Brain Tumor
Drug Kinetics
The drug undergoes a rapid conversion at a physiologic pH to active compound. In order words, temozolomide is not active directly. There are four basic ways in which the drug is controlled in the body. These include absorption, metabolism, distribution and elimination. The drug control processes are necessary information in order to determine appropriate dose in patients with reduced body organ function i.e. kidney failure. In this article, I will only address absorption and excretion.
Dosing and Administration
The two conditions for which temodar are prescribed are treated below with their corresponding respective dosage:
For newly diagnosed glioblastoma multiforme, administer 75mg/m2 for 42 days together with focal radiotherapy followed by initial maintenance dose of 150mg/m2 once daily forDays 1-5 of a 28 day cycle of temodar for 6 cycles.
For refractory anaplastic astrocytoma, the initial starting dose is 150mg/m2 once daily for 5 days per 28 day management cycle.
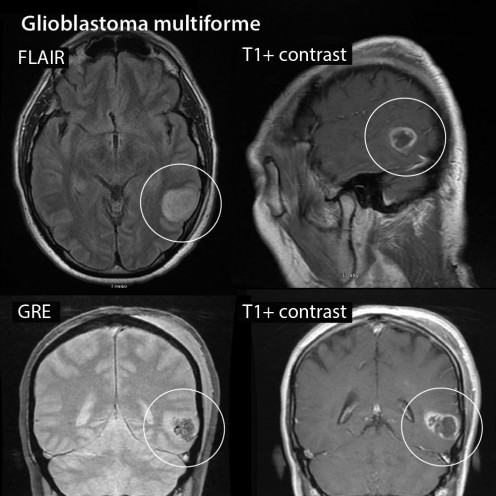
Glioblastoma Multiforme
Drug Absorption
After oral administration, temozolomide is rapidly and completely absorbed with highest level occurring at 1 hour. Certain drugs are affected by food intake and this in turn, can affect the time of action of the drug. Unfortunately, temodar happened to be one of those types of drugs. In fact, food reduces the rate and extent of its absorption. A certain study found that oral capsule and intravenous form of the drug infused over 90 minutes have similar absorption rate. Therefore, the oral formulation is considered bioequivalent to the injection.
Excretion
It is essential that a drug undergoes excretion process in order to avoid toxicity. About 38% of administered temozolomide dose is recovered over 7 days. In, fact, the major route of excretion of the drug is via the kidneys. If an individual has kidney problem, then consideration is given in dosing the medication for appropriateness. The primary elimination pathway for the drug is by pH-dependent degradation to a carboxamide.
Intravenous Temodar Formulation
Temodar Capsule
Side Effects
Some of the side effects attributed to the administration of temodar include headache, nausea, vomiting, and loss of appetite, constipation and weakness. Nausea and vomiting can sometimes be quite serious. Food and Drug Administration mandates that every manufacturer must continue to conduct post marketing surveillance to ensure public safety. Sometimes, the effects are managed when not serious since the risk of the tumor is higher. Therefore, one weighs benefit versus risk.
Conclusion
As new scientists continue to emerge, the world would continue to have answers to challenging medical dilemma. Brain tumor is not an easy scientific problem to address, but a very difficult and challenging one. I hope drug manufacturers specializing in oncology therapeutics would continue to advance the training in drug development. Temozolomide is a miracle drug for brain tumor due to its availability in oral and intravenous forms.