The Conspiracy to Deny Americans Affordable Prescription Drugs
If you navigate to any big drug company’s website, you will find, very quickly, a dozen or so patient assistance programs that a drug company offers to patients who need help paying for their medications. However, if you navigate to that same drug company’s website based in a foreign country, you're likely to find that these patient assistance programs don't exist.
Why?
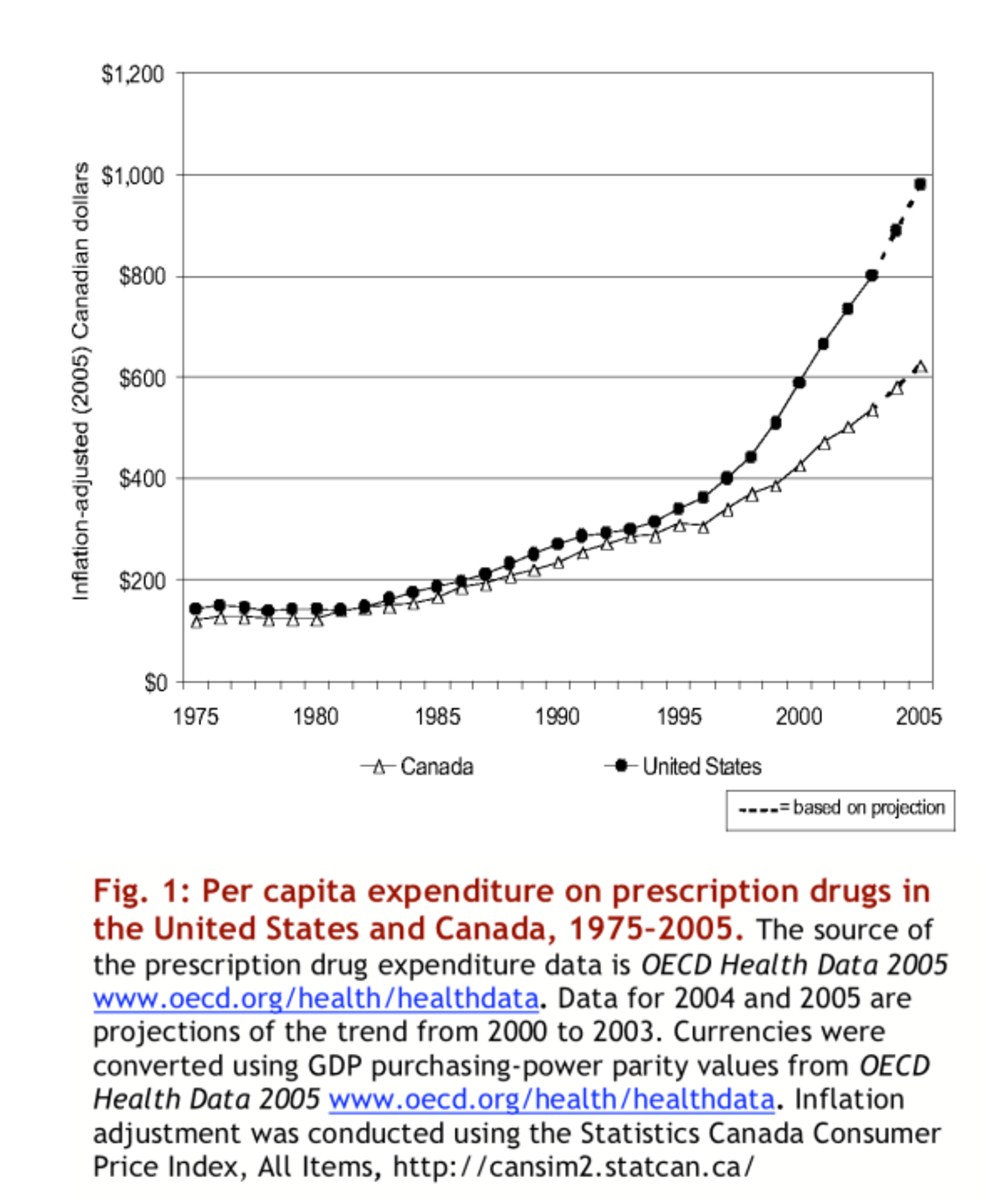
In the United States, drug companies are allowed to determine the cost of prescription drugs. In contrast, foreign countries have governmental agencies that oversee the cost of prescription drugs. Although opponents of price regulation for prescription durgs often argue that this would cause drug companies to stop offering its products in the U.S., this doesn't appear to be a problem in foreign countries, and as a matter of fact, many Americans have often looked to these foreign countries to obtain their prescription medications at an affordable price.
Under the Federal Food, Drug and Cosmetic Act, Congress granted authority to the FDA to oversee the safety of medications, and this included a suggested policy of lax enforcement for Americans who imported prescription medication, and specifically from Canada, for personal use.
John Horton's position under the Bush Administration, along with his policy suggestions, enabled him to launch a multi-million dollar business:
John Horton, founder of Legit Script, served as the primary policy advisor to Bush Drug Czar, John Walters, at the Office of National Drug Policy (ONDCP) from 2002-2007.
John Horton's Time Line of Conspiracy
Enter, John Horton
Beginning around 2003, however, the FDA began to crackdown on Canadian pharmacies that were shipping prescription drugs to American consumers via warning letters that threatened legal action against the pharmacies and the employees.
Despite wide acceptance that Canada's regulations are similar to America's...

The FDA sent dozens of warning letters to Canadian pharmacies

Google's role in Canadian prescription drugs
In 2004, Google and other search engines were notified by the U.S. government that a better monitoring system for pharmacies needed to be implemented. Google eliminated all foreign country pharmacies from advertisements, but did continue to allow Canadian-based pharmacies to advertise. Google contracted with Pharmacychecker to verify the integrity of online pharmacies.
In retaliation, drug companies stopped filling orders to Canadian pharmacies that were shipping its products to Americans. This caused drug shortages in Canada and other countries around the world.
Pfizer was the first company to issue letters to Canadian pharmacies, threatening that if shipments were to continue to America, supply would be cut off. Other drug companies followed suit.
Canada reports on blacklisting in 2003, and the U.S. reports on blacklisting in 2004.
- Blacklist may force Net druggists to close - Winnipeg Free Press
Last week, Pfizer Inc, the world's largest drug manufacturer, blacklisted 50 Canadian pharmacists. Pfizer supplies popular medications for arthritis, blood pressure, cholesterol and sexual dysfunction, including Viagra. - Pfizer Moves to Try to Stop Drugs From Canada - New York Times
Pfizer is stepping up its efforts to stop exports of low-price prescription drugs from Canada to the United States by imposing new restrictions on sales to Canadian drug wholesalers.In a letter to
Drug-giant Pfizer says importing from Canada is legal!
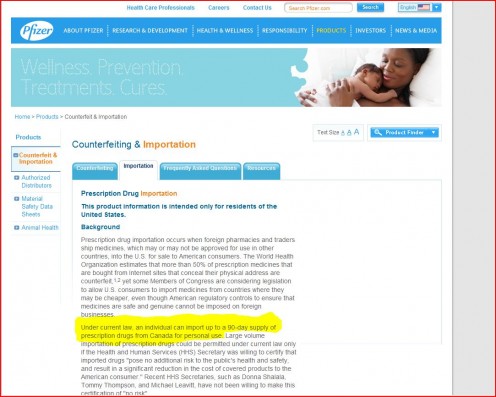
It's not about safety, because safety measures are already in place:
In 1999, the National Association of Boards of Pharmacy created an online pharmacy accreditation program, VIPPS. This accreditation is given to online pharmacies that adhere to state and federal regulations. In 2003, NAPB began to administer the program in Canada and is responsible for accrediting Canadian pharmacies. Canadian pharmacies that ship prescription drugs to America are not able to receive accreditation.
State-run, Canadian prescription programs shut down
Several states enacted legislation, beginning in 2004, that would have allowed residents to obtain prescription drugs from Canada. These programs, however, were either shut down shortly after being launched or were never launched at all. The FDA immediately sent letters to the governors of each state threatening legal action. Some of these states were:
Wisconsin
In 2004, Wisconsin approved legislation that would allow residents to obtain prescription medications from Canada. The website that was set up for Wisconsin residents, www.drugsavings.wi.gov no longer exists and is currently for sale.
Second letter from FDA to Governor of Wisconsin:
http://www.fda.gov/Drugs/DrugSafety/ucm179517.htm
Washington
In 2005, legislation was enacted allowing residents to obtain prescription medications from Canada under the condition that the FDA authorize a waiver for all residents. This waiver was denied by the FDA:
http://www.fda.gov/Drugs/DrugSafety/ucm179358.htm
Currently, Washington's Insurance Commissioner has a booklet available for residents for information on paying for prescription drugs. On page 11 of the booklet, last updated 11/2010, it outlines the inconsistencies of laws and policies by governmental agencies.

Texas
Governor Rick Perry of Texas was the recipient of a warning letter from the FDA when he signed legislation allowing residents to obtain prescription drugs from Canada. The letter addressed FDA's policy for personal use, stating it doesn't apply to a commercialization of an entire state:
However, this policy is not intended to allow importation of foreign versions of drugs that are approved in the U.S. , particularly when the foreign versions of such drugs are being "commercialized" to U.S. citizens.
Texas's Attorney General was also the recipeint of a warning letter from the FDA:
FDA retains the authority to bring an enforcement action in any case in which a provision of the FFDCA has been violated.
Potential Liability
There are many sources of civil and criminal liability for parties who violate the FFDCA.
Nevada
The state of Nevada, in 2005, passed legislation allowing residents to obtain prescription medications in Canada.
The FDA sent a warning letter to Governor Kenny Guinn indicating that if Nevada's Board of Pharmacy were to license any Canadian pharmacy, Nevada would be in violation of federal law:
The licensure of Canadian pharmacies by the State of Nevada Board of Pharmacy will not only result in violations of federal law, but may put your citizens at risk.
The FDA later sent a warning letter to Nevada's Board of Pharmacy warning that any licensure of a Canadian pharmacy would result in violating federal law and Nevada's own laws:
We note that the Nevada law states that pharmacies, to be licensed, must comply with all applicable federal laws, regulations and standards. Sales by Canadian pharmacies that are licensed by the Nevada Board of Pharmacy to sell unapproved drugs to Nevada residents would violate the federal FD&C Act. Thus the licensing of such pharmacies will lead to actions in violation of your law.
In 2006, Nevada's legislation was vastly amended to include that it is unlawful to obtain a prescription from a Canadian pharmacy that does not have a license in the state of Nevada. The prescription drug program was, effectively, scrapped.

National Association Boards of Pharmacy recommends prosecuting individuals
In 2004, a U.S task force was created to explore the possiblity of allowing Americans to import medications from Canada and other countries. Many of the governors who enacted legislation testified before the committee outlining safety measures, oversight and compliance that was put into place for the state programs.
The task force did not recommend approving any of these programs.
Carmen Catizone, Executive Director of the NABP (National Association of Boards of Pharmacy), did, however, suggest that individuals importing medication be the target of enforcement. And this is what began to happen in 2006.
- More Medicines From Abroad Seized - Los Angeles Times
The U.S. government apparently is stepping up seizures of cheap drugs ordered by Americans -- mainly seniors -- from abroad, Canadian pharmacies say.The pharmacies, which sell drugs by mail and over - Seized Drugs Being Released - Los Angeles Times
Amid mounting criticism of their crackdown on mail-order medications, customs officials have begun releasing hundreds of seized packages to consumers, Canadian pharmacies and U.S. lawmakers said
In 2006
After receiving phone calls from constituents complaining their prescription medications had been seized by the U.S. government, members of Congress wrote a letter to the FDA demanding an explanation as to why the personal use policy had changed. The FDA denied that its policies had changed and released the seized medications.
In 2007, John Horton Launches Legit Script
According to Pharmacychecker.com, John Horton registered his company's domain (Legitscript.com) while he was still employed with the Office of National Drug Policy (ONDCP) in March of 2007. Pharmacychecker has a snapshot of the registration date.
In April of 2007, John Horton launched his company, Legitscript. However, his profile on LinkedIn has been updated to reflect Legitscript was launched in May. This, after an ethics violation complaint from Pharmacychecker to Darrell Issa.
Strategy

Before John Horton left his position in Washington, he made sure that Pharmacychecker's reputation was trashed:
In April of 2007, Director of the Office of National Drug Policy, John Walters--who John Horton advised--, submitted a strategy to stop rogue pharmacies, counterfeit drugs and the importation of prescription drugs. His strategy included an analysis of Pharmacychecker, and he concluded that it was not an "adequate, reliable verification system", also mentioning that Pharmacychecker was not recognized by the National Association Boards of Pharmacy.
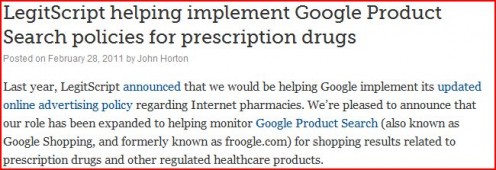
When Google didn't terminate its contract with Legitscript, Google was investigated by the U.S. Government
In 2009, Pharmacychecker received a subpoena by the Office of Criminal Investigations to produce documents relating to Google's advertising policies for online pharmacies.
In January of 2010, Google terminated Pharmacychecker's contract.
In April of 2010, Legitscript was awarded a contract by Google to monitor all online pharmacy advertisements and to ensure that the ads are VIPPS accredited (or endorsed by Legitscript). In February of 2011, the contract was expanded to include other services.
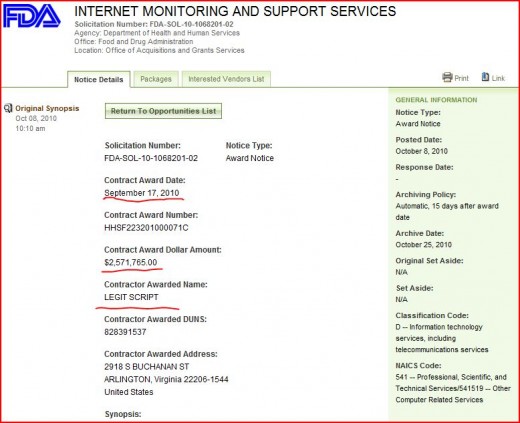
In September of 2010, Legitscript was awarded a $2.5 million dollar contract by the FDA to monitor internet search results and the advertising of online pharmacies.
- Google Near DOJ Settlement Over Online Drug Ads - WSJ.com
Google is close to settling a U.S. criminal investigation into allegations it made hundreds of millions of dollars by accepting ads from online pharmacies that break U.S. laws. - August 24, 2011: Google Forfeits $500 Million Generated by Online Ads & Prescription Drug Sales
August 24, 2011: Google Forfeits $500 Million Generated by Online Ads & Prescription Drug Sales by Canadian Online Pharmacies
Don't mess with the U.S. Government or previous employees of the White House
In August of 2011, Google agreed to forfeit $500 million dollars to the U.S. government for its role in enabling American consumers to purchase prescription medications from Canadian pharmacies. The forfeiture represents gross revenue from advertising AND gross revenue in sales to Canadian pharmacies.
Currently, the NABP endorses Legitscript as another alternative for consumers to verify online pharmacies.
What's a little competition? Competition cost Google $500 million dollars

John Horton's policies while employed in Washington under the Bush Administration helped him launch his company, ensuring that his services would be needed. It also ensured him success, as the contacts he made during his time in Washington paved the road for government contracts.
What this boils down to for American consumers
John Horton, single-handedly, is responsible for the advertisements you see displayed on any website regarding online pharmacies and prescription medications. It also means that search results popping up for you when searching for medical information are, largely, results that John Horton wants you to see.
If you want to bypass John Horton and his million-dollar contracts with the government when you are looking for medical information, you might want to think about utilizing Google Canada or Google UK.
This also means that if you happened across this article in search on Google USA, John hasn't seen it yet.
Hi, John...You Suck, big time!