Toxins, Xenobiotics, Drug interactions and ways to improve detoxification processes

Xenobiotics and Total load [1]
- Xenobiotics refer to chemicals or molecules foreign to living organisms [1; page 237]
- The action of toxic agents on the organism (on its structure and function of its molecules) can occur at or within multiple sites which includes the functional cellular components (e.g. active transport mechanisms), receptors, enzymes and nucleic acids [1].
- The toxicant/toxic agent can make it difficult for the organism to properly carry out essential functions such as absorption, distribution/solubility, metabolism and excretion of the toxicant itself
- An example involves a toxic agent that diminishes the functional capacity of the liver or kidneys to metabolize and excrete that toxicant, it can become increasingly toxic as functional capacity decreases
- The rate of distribution and tissue accumulation of a substance will affect the toxicity of that substance relative to organ/tissue structure and function
- Need to look at chemical substances and the role of nutrients in protecting the body against these xenobiotics and promoting elimination of these toxicants
Total Load [1; page 238]
- The key lies in understanding how xenobiotics affect human health
- Total load = describes the total of all exposures and influences that bear on human physiology; these factors often determine the efficiency of the body’s detoxification system, nutrient status and organ reserve and are central to how xenobiotics affect humans
- The concept of total load suggests that the sum of the factors may overwhelm an individual’s system of metabolic management
The following factors influence the total load phenomenon [1; page 239]:
- Xenobiotics (insecticides, herbicides, drugs, solvents, metals, etc)
- Infections (streptococcus, pseudomonas, parasites etc)
- Toxicants (aflatoxin, fumosine, penicillum toxins, ergot toxins, etc)
- Biological inhalants (molds, algae, pollens, foods, etc)
- Physical phenomena (electromagnetic fields, ionizing radiation)
- Lifestyle (drinking, smoking, etc)
- Mechanical problems (biochemical dysfunction, such as nasal, intestinal and other obstruction)
- Hormonal aberration (DHEA, cortisol, estrogen, progesterone, testosterone, etc)
- Psychosocial factors (stress, coping skills, belief systems, psychological trauma)
Even though nutritional status is not a direct part on total load, the above factors are widespread and influenced by nutritional status [1].
Total load and nutrient metabolism are inseparable components of any program designed to manage the physiological alteration that occurs with chemicals [1].
Heavy metal toxicity
-For a long time, efforts to understanding how xenobiotics affect human health focused at determining whether or not a substance produced cancer in laboratory animals [1]. Although this gives us insight on detoxification, metabolism and cell biology, nowadays it is important to look at the capacity of xenobiotics at altering the function of biological systems and how this can contribute to illness because cancer (an end-stage manifestation of chemical exposure) seems like a secondary problem as many physiological events precede cancer development [1].
-Chronic subacute exposures may lead to subtle or overt long term problems in certain individuals [1]
Two important issues have been illustrated by “Environmental Neurotoxicology” and “Biological Markers in Immunotoxicology” as follows:
*Functional changes result from low chemical exposure and their effects can multiply when one is exposed to more than one agent [1].
- Although environmental exposure to toxic metals may be highly variable, evidence suggests that toxic elements directly influence behaviour by impairing brain function, influencing neurotransmitter production and utilization and altering metabolic processes [1]
- One way researchers can gain meaningful information about the toxic load of a patient who may be experiencing cumulative toxic intake and exposure over time is through hair element analysis [1]
- The multiple mechanisms if toxicity include enzyme/cofactor inhibition, enzyme potentiation, disruption of membrane and other transport processes and a weakened neuronal functioning or nerve conduction processes [1]
- The extent at which these toxic elements and associated adverse effects affects people varies among individuals based upon biochemical individuality [1]
- Biochemical individuality helps explain different reactions to toxic element exposure [1]
Mercury Toxicity; known as Minamata disease [1; pages 244-245] and potential toxicity in light of biochemical individuality
Biochemical individuality;
-The disease was originally called Congenital Minamata disease until researchers observed that the offspring of symptom-free patients suffered paralysing neurological effects [1]
-Due to biochemical individuality, not every victim of toxic element poisoning experiences all symptoms and deviations to the same extent; an example is illustrated in the fact that as little as 5 parts per million (ppm) may be associated with mercury toxicity whereas victims of Minamata disease have a concentration of 183ppm [1]
-The toxicity of mercury involves both tissue destruction and enzyme inactivation. Excess mercury results in pronounced toxicity and chronic insidious disease conditions such as autoimmune disease [1]
Signs and Symptoms of Mercury (Toxic element exposure) [1; page 245]
- Reduced sensory abilities (taste, touch, vision and hearing)
- Metallic taste with increased salivation
- Fatigue
- Anorexia
- Irritability and Excitability
- Psychoses
- Mania
- Anemia
- Paraesthesias
- Tremors and incoordination
- Increased risk of cardiovascular disease
- Hypertension with renal dysfunction
Ways to gain information on the toxic load of a patient:
*Hair element analysis
*Urine analysis
Functional Approach to Toxicity
- Assess relationships among toxicants, toxic load and clinical manifestations are critical [1]
- Decrease toxic load is an immediate consideration when dealing with toxicity (look at exogenous and endogenous sources of toxins);
- Assess Exogenous sources using a questionaaire/interview with the patient; aim to incorporate lifestyle approaches to minimizing exposure
- Assess Food allergies (Food Allergy Assessment)
- Promote Bacterial Balance (bacterial flora) with prebiotics and probiotics and sometimes antimicrobials are warranted; assess gastrointestinal function
- Promote healthy detoxification to endogenous and exogenous harmful substances
Biochemistry of Detoxification and clinical relationships with individuals
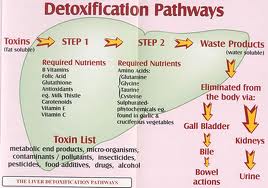
- 2 distinct phases exist ; known as Phase I and Phase II that chemically biotransform lipid-soluble substances into progressively more water-soluble substances (makes them excretable) through a series of chemical reactions
Phase 1 reactions:
*Phase 1 reactions usually involve oxidation, reduction or hydrolysis; a family of enzymes commonly referred to as cytochrome P450 mixed-function oxidases begins the process of detoxifying xenobiotics and endogenous substances [1]
*This system is a group of many isoenzymes that have specific affinity for differing substrates
*involves adding or exposing a functional group, most commonly a hydroxyl (OH), to the toxic molecule; this biotransformation allows the Phase I compound to undergo Phase II conjugation reactions.
*In some cases, the compound may be eliminated directly after the Phase I reaction but most commonly the Phase I reaction produces an intermediate that must undergo further transformation. These intermediates can be highly reactive and are often more toxic than the original compound [1]. This intermediate step in the transformation of toxic substances to excretable, harmless metabolites is called bioactivation
*A consequence of this biotransformation is an increase in free radical molecules. As a result, the more efficiently Phase II reactions to act on these intermediates, the less likely it is that tissue damage will occur from excess reactive molecules [1].
*the balance between Phase I and Phase II is critical to detoxification [1]
Phase II reactions:
*involves various bio-transformed molecules that are conjugated and involves distinct reactions [1]
*the main conjugation reactions are glucuronidation, amino acid conjugation, sulfation, glutathione conjugation, acetylation and methylation
*these conjugation reactions add a water-soluble molecule to the intermediate metabolite to further increase its hydrophilic (water loving) qualities which prepares the metabolite for urine or bile elimination [1]
Clinical Relationships with Individuals [1; page 252]
*Sluggish, imbalanced or impaired detoxification systems can result in the accumulation and deposition of metabolic toxicants, increased free radical production and it’s following pathology, impaired oxidative phosphorylation and reduced energy [1].
*Various nutrients are required for proper detoxification function
*Substances that upregulate Phase I such as smoking, alcohol and certain medications can badly affect this balance because Phase II pathways may be unable keep up with the increased toxic load
*Various medications like fluoxetine and H2 blockers (cimetidine) may inhibit Phase I [1]
*A good example was reported in a male in Texas who was exposed to low levels of lawn chemicals while taking the prescription drug cimetidine [1]. This exposure critically damaged his central and peripheral nervous system. He responded so severely to a low level of a toxic agent (the lawn herbicide) that cimetidine is concluded to be a CYP450 inhibitor which impaired his liver’s ability to detoxify compounds in the lawn treatment and led to permanent neurological damage [1]. This supports that the relative detoxification ability of an individual plays a vital role in the toxicity or carcinogenicity of a specific substance [1].
An example of drug nutrient interactions that may influence nutritional status.
In drug, diet, and patient variables, there are many considerations such as drug dose and duration, dietary fat intake, age, sex and genetics [1].
Drug-Nutrient Interactions can be classified according to the following criteria:
- Location (stomach, gallbladder, etc)
- Mechanism (chelation, precipitation, etc)
- Pharmacologic or nutritional outcomes (drug variables, diet variables)
- Drug or drug group (antibiotic, antacids)
- Nutrient (folic acid, pyridoxine, etc)
- Temporal relationship to food or nutrient digestion (effect of drug/food/nutrient interaction over time)
- Patient group affected (asthmatic, arthritic, diabetic, epileptic)
- Risk factors (laxative abuse, fasting, drug excess, etc)
Some examples of Drugs affecting Nutritional status
- Cimetidine may impair Vitamin B12 absorption by influencing acid secretion
- Bicabonate may increase pH and decrease folate absorption
- Drug type classification e.g. Tetracycline impairs absorption of Calcium, Magnesium, Iron and Zinc; also milk lowers tetracycline bioavailability
- Taking Cholestyramine and Vitamin A hinders Vitamin A absorption
Hence, practitioners should consider the potential of drugs to contribute to total toxic load and be aware of drug-nutrient interactions and how they influence metabolic status of a patient [1].
Both Phase I and Phase II detoxification reactions contain a nutritional component, enzymatic cofactors and nutrients. What is the facet of detoxification-how is nutritional help obtained and from where?
Regulation of Phase I and Phase II activity levels has a dietary component [1; pages 256-257].
*Nutritional support of Phase I and Phase II reactions involves administering increased amounts of enzymatic cofactors and other nutrients involved in cytochrome P450 enzymes [1].
Detoxification support nutrients: Vitamins B2 (riboflavin), B3 (niacin), B6 (pyridoxine), B12 (cobalamin) and folic acid. Tripeptide glutathione and the branched-chained amino acids leucine, glycine, isoleucine and valine. Flavonoids and phospholipids
Protective antioxidant support (to handle reactive oxygen intermediates produced during Phase I activity) includes:
*carotenoids including beta-carotene (pro-vitamin A), ascorbic acid (Vitamin C), the tocopherols (Vitamin E) and the coenzyme Q10 (ubiquinone)
* Antioxidant minerals (selenium, zinc, copper and manganese).
*Thiol compounds (found in garlic, onions and cruciferous vegetables, flavonoids, silymarin and anthocyanidins
Nutritional Phase II activity support includes:
*Wide variety of sulphur-containing compounds that serve as sulphur donors in the sulphate conjugation process
*Includes the amino acids; cysteine, N-acetyl-cysteine, methionine and taurine
*Inorganic sulphates
*Other amino acid conjugation pathways require: glycine, glutamine, ornithine and arginine. Glucuronic acid and glutathione are necessary in their respective conjugation pathways
Is there a link between detoxification and oxidative stress. If so, how is it managed?
*Some drugs, xenobiotics and other substances seem to uncouple electron transport and oxidative phosphorylation [1]. Without proper detoxification, the propensity for oxidative stress increases [1]. These exogenous influences may lead to disease through altering mitochondrial function such as the exposure to toxins like 3-nitropropionate, a fungal toxin found on the sugar cane, can cause ozidative phosphorylation to uncouple and hence initiating mitochondrial oxidative stress and ultimately neuronal death [1].
*Certain antibiotic drugs like doxycycline, imipenem, and leucinostatins A & B can also uncouplemitochondrial oxidative phosphorylation and increase oxidative stress [1].
3 molecules in the mitochondrion protect the membrane and other components from oxidant damage:
-Lipoic acid (a sulphur containing organic acid) 50-1000mg/day
-Coenzyme Q10 (the non-protein component of the ETC) 50-1000mg/day
-Glutathione (a tripeptide formed from glutamate, glycine and cysteine) 100-1000mg/day
Specific nutrients that improve mitochondrial efficiency:
-Ascorbate 500-6000mg/day
-Catechin 50-1000mg/day
-Copper 1-3mg/day
-Vitamin E 2000iu/day
-B complex vitamins
-Vitamin K3 and K1
-L-carnitine
-Coenzyme Q10 20-1000mg/day
-N-acetylcysteine and/or glutathione
-Creatine
Approach a clinician may take to help support a patient’s detoxification system.
Firstly, assessment of detoxification is paramount. The body’s detoxification systems are very complex and show a great amount of variability and are extremely responsive to an individual’s environment, lifestyle and genetic uniqueness [1].
Some ways to assess detoxification are:
*Genetic test (that determines if someone is a fast or slow metabolizer)
*Genetic tests are available for assessment of Phase I detoxification enzymes and are becoming increasingly used in association with narrow spectrum drugs or to assess propensity for certain cancers
*A challenge test (whereby a patient is given a known amount of a substance not normally in their body e.g. acetaminophen and then obtaining urine over a specified time period and then assessing how much of the different metabolites of that substance has been excreted)
These tests give us a thorough picture of how well a person is detoxifying exogenous substances [1].
Functional Approach to Toxicity
- Assess relationships among toxicants, toxic load and clinical manifestations are critical [1]
- Decrease toxic load is an immediate consideration when dealing with toxicity (look at exogenous and endogenous sources of toxins);
- Assess Exogenous sources using a questionaaire/interview with the patient; aim to incorporate lifestyle approaches to minimizing exposure
- Assess Food allergies (Food Allergy Assessment)
- Promote Bacterial Balance (bacterial flora) with prebiotics and probiotics and sometimes antimicrobials are warranted; assess gastrointestinal function
- Promote healthy detoxification to endogenous and exogenous harmful substances
REFERENCES
1) DeAnnLiska, Sheila Quinn, Dan Lukaczer et al.Clinical Nutrition-A Functional Approach, 2004, The Institute of Functional Medicine, a Nonprofit educational organization