Examining Tysabri (Natalizumab) for the Relapsing-Remitting Form of Multiple Sclerosis
This paper discusses the use of Natalizumab (Tysabri) for the treatment of the relapsing/remitting form of Multiple Sclerosis, a chronic neurological disease that affects the central nervous system by destroying the myelin surrounding the axons of neurons. Relapsing/Remitting Multiple Sclerosis is the most common of four recognized forms of Multiple Sclerosis. The use of Tysabri was intended to reduce the relapse frequency, in turn decreasing the reoccurrence of symptoms. After the approval of Tysabri the FDA announced a warning considering its suspension due to reports of adverse side effects.
This paper was originally written and released in 2006.
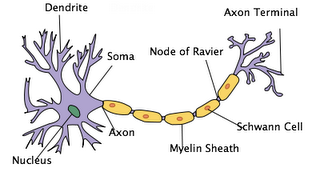
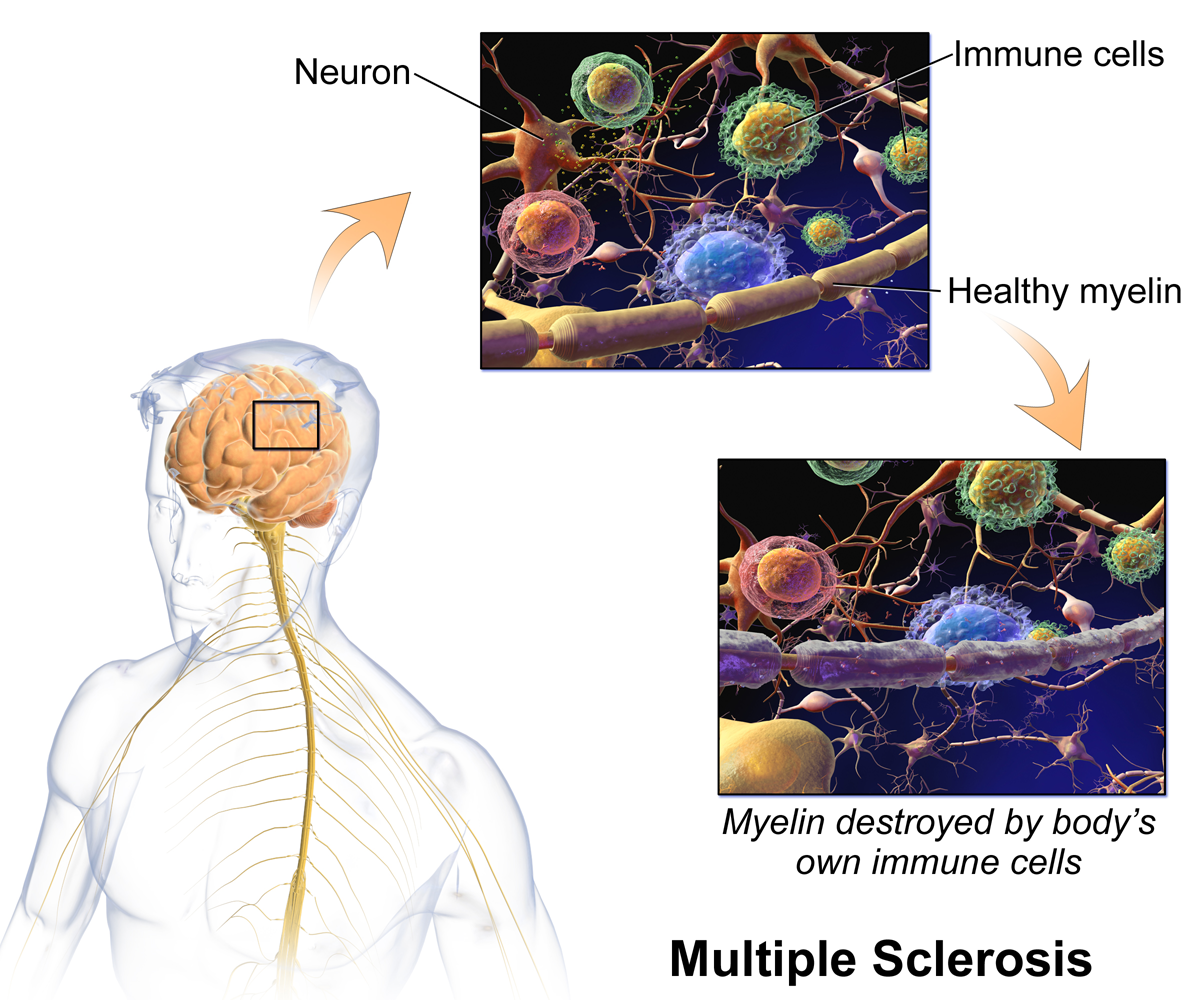
Multiple Sclerosis (MS) is thought to be an autoimmune disease in which the body’s own immune system attacks a tissue or organ of the body (Alsleben, Donsbach, Kalb, et al., 1996). In MS, the immune system attacks the myelin of the central nervous system. The myelin, which is made and maintained by glial cells called oligodendrocytes, is a fatty substance that forms a protective sheath around nerve fibers. The myelin helps to make the conduction of nerve impulses possible. Damage to the myelin, caused by MS, results in a disruption in the transmission of nerve impulses, or neural messages, analogous to a damaged telephone wire or cable (Kalb, 1996). This is because neurons with myelinated axons develop sodium channels mostly at the nodes of Ranvier, which are interruptions in the myelin formed at intervals of approximately 1mm along the axon (Kalat, 2004). When the axon begins to lose its myelin, the areas that were previously covered with it are deficient in sodium channels, therefore inhibiting action potentials from traveling along the axon. Wherever myelin is destroyed, a plaque (lesion) forms, with a gradual build up of scar tissue (sclerosis) at the site. These scarred sites occur in multiple locations, and for that reason it is referred to as Multiple Sclerosis (Kalb, 1996).
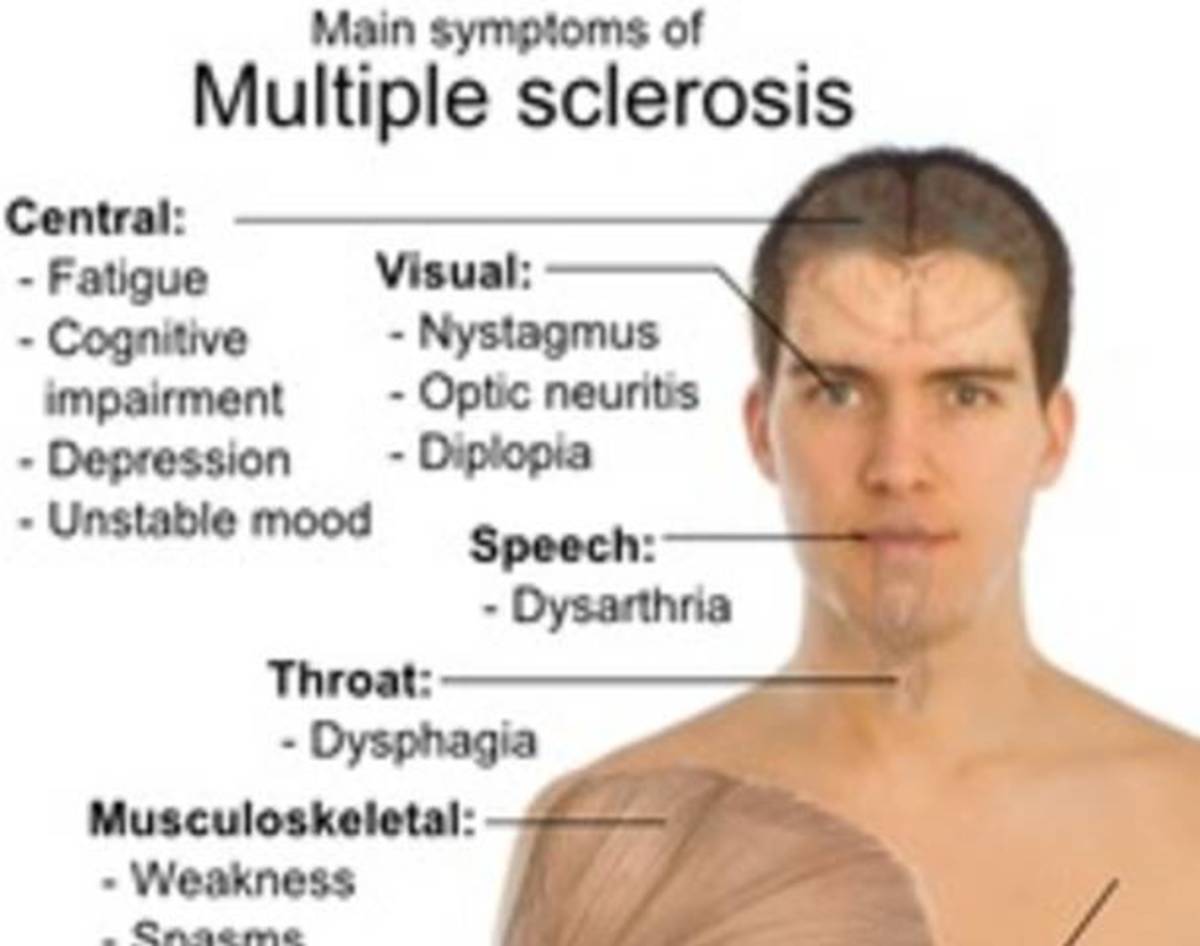
The National Multiple Sclerosis Society (2005) describes the relapse/remitting form of Multiple Sclerosis as the most prevalent of the four recognized forms of the disease. Characteristics of the relapse/ remitting form include symptom flare-ups (relapses), followed by periods of partial or complete recovery (remission) from symptoms. During a relapse, the myelin is damaged by the formation of a new plaque or the reactivation of an old one (Kalb, 1996). These periods of relapse can cause a variety of symptoms to erupt including loss of or double vision, stiffness, weakness, emotional changes, and fatigue among others. Several treatments have been used to help lessen the occurrences and severity of symptoms (Alsleben, Donsbach, Kalb, et al., 1996). One common treatment method is physical therapy, which may be used to improve functional ability and prevent debilitating complications. Other treatments have included the use of adrenocorticotropic hormone (ACTH), and drugs such as interferon beta 1-a (Avonex), oral myelin, and transforming growth factor beta.
In 2004, the Food and Drug Administration (FDA) approved the first monoclonal antibody treatment for Multiple Sclerosis known as natalizumab, or Tysabri. Unlike other drugs that are used to treat Multiple Sclerosis, Tysabri is a monoclonal antibody that is given intravenously every four weeks (National Multiple Sclerosis Society, 2005). A monoclonal antibody is defined as an antibody that is produced artificially from a single cell clone and consists of a single type of immunoglobulin, which is a class of globulin proteins that function as antibodies (NASA, 2005). The purpose of Tysabri is to prevent the movement of damaging immune cells from the blood through the blood-brain barrier (BBB) and into the brain and spinal cord. The blood-brain barrier is composed of endothelial cells joined together tightly, forming a wall that keeps out most chemicals, viruses, and bacteria (Kalat, 2004). Tysabri crosses the BBB by attaching to alpha 4-integrin, a protein on the surface of T cells, that enables it to pass through (National Multiple Sclerosis Society, 2005). This function is known as active transport, which is defined as a protein mediated process that expends energy to pump chemicals from the blood into the brain (Kalat, 2004).
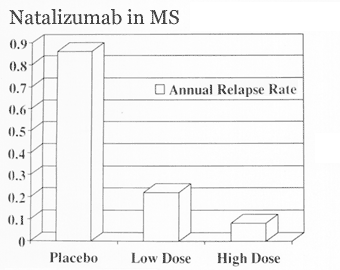
The approval of Tysabri to be administered and distributed was based on two ongoing two-year clinical studies conducted by Biogen Iden and Elan Corporation that involved more than 2,100 patients with MS (Wakine, 2004). In one trial (the AFFIRM trial) patients were given either Tysabri or a placebo, and in the other study (the SENTINEL trial) patients already receiving interferon beta-1a treatment were given Tysabri in combination. In both studies, Tysabri was shown to significantly reduce the incidence of relapse (National Multiple Sclerosis Society, 2005 & Food and Drug Administration, 2004). The AFFIRM study showed a 66% reduction in relapse rate for those taking Tysabri. In addition, compared to those receiving a placebo, Tysabri recipients had approximately 40% fewer new MRI lesions. Table 1 illustrates the results of the AFFIRM study, comparing relapse rates for those taking Tysabri versus those receiving a placebo. The results display the significant effect Tysabri had on minimizing the incidences of relapse.
For the participants receiving Tysabri in the SENTINEL study, 54% experienced a reduction in relapse rate compared to those receiving a placebo in combination with Avonex. In addition, 67% of patients in the SENTINEL study receiving Tysabri developed no new or enlarging MRI lesions compared to 40% of the placebo group.
The National Multiple Sclerosis Society (2005) released a report documenting the findings of the clinical studies conducted on the use of Tysabri. According to their report, Tysabri caused only minor side effects including headache, fatigue, urinary tract infection, depression, sore throat, and limb and joint pain. Furthermore, the incidence of infection was similar to those participants receiving a placebo.
Once Tysabri had been approved for marketing, patients who were prescribed Tysabri were to receive the drug intravenously at either a doctor’s office or other medical facility. The wholesale cost per vial (dose) was priced at $1,808, resulting in a cost of $23,504 a year if taken every four weeks (National Multiple Sclerosis Society, 2005).
Shortly after Tysabri was approved for marketing, the Food and Drug Administration (2005) released a public health advisory concerning the suspended marketing of Tysabri and further dosing by physicians, following accounts of serious adverse effects reported with its use. The FDA received a report from the manufacturer of Tysabri, Biogen Idec, of one confirmed fatal case and an additional case of one patient developing Progressive Multifocal Leukoencephalopathy while receiving the drug. Progressive Multifocal Leukoencephalopathy (PML) is described as an infrequent disorder of the nervous system that primarily affects individuals with suppressed immune systems and is characterized by demyelination (destruction of myelin) (National Institute of Neurological Disorders and Stroke, 2005). Symptoms include mental deterioration, vision loss, speech disturbances, inability to coordinate movements, paralysis, and in rare cases, seizures. There is currently no cure for PML and its course is progressive with the outcome of death usually occurring between one and four months after onset.
According to the Food and Drug Administration (2005), there have been no previous occurrences of PML developing in patients taking Tysabri. The patient who died and the patient who developed PML had both been enrolled in a long-term clinical trial and were taking Tysabri for more than two years. Furthermore, neither patient had any known risk factors for PML and both patients were also receiving interferon beta-1a (Avonex), demonstrating the possibility that the onset of PML may have been due to taking Tysabri in combination with Avonex, or a number of other factors.
Although the exact relationship between Tysabri and PML is unknown, the FDA has announced the volunteer suspension of the drug by Biogen Idec, who also agreed to notify all patients who had received it of any possible risks (FDA, 2005). They offer no advice to patients at this time other than to discontinue use, although Biogen Idec, the FDA, and other scientific experts have begun to assess the possible relationship between Tysabri and PML. Aside from the two reported incidents, the use of Tysabri has thus far proven to provide a beneficial use for patients. With further studies considering the relationship between Tysabri and PML results may assist in indicating the causes for any relationship and, with modifications to the treatment of Tysabri for MS, prevent any reoccurrences of PML.
Further Reading
Wikipedia Page for Multiple Sclerosis
http://en.wikipedia.org/wiki/Multiple_sclerosis
National Multiple Sclerosis Society
http://www.nationalmssociety.org/index.aspx
Tysabri Website
http://www.tysabri.com/
References
Alsleben, R. & Donsbach, K.W. (1993). Multiple Sclerosis, Muscular Dystrophy, and ALS. Delaware: Rockland.
Food and Drug Administration. (2004). First Monoclonal Antibody Treatment for Multiple Sclerosis Approved. Retrieved Feb 2, 2005, from http://www.fda.gov/bbs/topics/news/2004/NEW01141.html
Food and Drug Administration, (2005). FDA Public Health Advisory: Suspended Marketing of Tysabri (natalizumab). Retrieved March 17, 2005, from http://www.fdo.gov/cder/drug/advisory/natalizumab.htm
Kalat, J. (2004). Biological Psychology. Belmont: Wadsworth
Kalb, R.C. (1996). Multiple Sclerosis: The Questions You Have the Answers You Need.New York: Demos Vermande.
NASA. (2005). Neurolab: Monoclonal Antibody. Retrieved March 5, 2005, from http://neurolab.jsc.nasa.gov/glossim.htm
National Institute of Neurological Disorders and Stroke. (2005). Progressive Multifocal Leukoencephalopathy Information Page. Retrieved March 19, 2005, from http://www.ninds.nih.gov/ disorders/pml/pml.htm
National Multiple Sclerosis Society. (2005). Companies Release Results of Completed 2-Year Trial of Tysabri in MS. Retrieved March 5, 2005, from http://www.nationalmssociety.org/Research-2005Feb17-2.asp
Waknine, Yael. (2004, Dec 2). FDA Approvals: Tysabri, Nexium, CR002, Sotradecol. Article 495203. Retrieved Febuary 2, 2005, from http://www.medscape.com/viewarticle/ 495203?src=search