Understanding Diabetes, Syndrome X and Ways to Control Blood Sugar
Overview of the problem of dysglycemia, problems associated with insulin resistance and the macronutrient balance
• Dysglycemia can be viewed as both a trigger and a mediator of chronic inflammation
• Dysglycemia is an imbalance that can be approached from a unified standpoint with interventions which will simultaneously reduce its potential as an inflammatory trigger and help return glycemic control as a mediating mechanism to the natural adaptive control of the body [1]
• There are 4 fundamental links between dysglycemia and the triggering of chronic inflammation; namely:
• -Syndrome X
• -Advanced Glycosylation End Products (AGEs)
• -the pentose phosphate pathway
• -Steroid metabolism
(1) Syndrome X
*Clinical Aspects of Syndrome X:
*Hypertriglyceridemia (greater than 150mg per decilitre); Dyslipemia (low HDL, elevated LDL)
*Hypertension
*Hyperuricemia
*Marginally elevated 2 hour post-prandial glucose (top 5%); Elevated serum insulin on a 2 hour glucose tolerance test
*Reaven has estimated that about 1 out of every 4 non-obese individuals with normal glucose tolerance (measured by 2 hour oral glucose tolerance test) has functional insulin resistance in which hypersecretion of insulin is required to maintain normal blood sugar levels
*Adipose tissue is highly sensitive to insulin concentration and small increases in circulating insulin can greatly increase plasma free fatty acid (FFA) levels-> When elevated FFA levels reach the liver, increase oxidation is stimulated -> this event may in itself increase risk of inflammatory response in relationship to upregulated oxidative metabolism (which may not be met with adequate nutritional support)
(2)AGEs and Inflammation
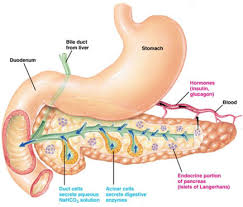
• In tissues that can take up glucose without the mediation of insulin (e.g. nerves, eyes, kidneys), intracellular concentrations of glucose can become excessive under hyperinsulinernic conditions
• Excessive glucose concentrations within these tissues can promote formation of advanced glycosylation end products or AGEs
• AGEs have a strong propensity to form crosslinks and to generate reactive oxygen intermediates that can damage cell structure. The mechanism for this process has been shown to involve receptors for AGEs (RAGEs) which bind to AGEs, stimulate monocyte migration, activate monocytes and trigger release of pro-inflammatory cytokines
(3)Pentose Phosphate Dysregulation and Inflammation
• This is the third area of linking dysglycemia with inflammation; involves the pentose phosphate pathway (PPP) otherwise known as the hexose monophosphate shunt (HMS)
• This pathway is critically linked to oxidative stress and inflammation since one of its key roles involves production of reducing equivalents in the form of NADPH
• NADPH reducing equivalents are required in the body for synthesis of fatty acids and for regeneration of the key antioxidant nutrient glutathione (GSH)
• When insulin levels in the body are normally high, increased uptake of glucose into the cells is expected and the rate limiting enzyme in the HMS, glucose-6-phosphate dehydrogenase (G6PD) is activated
• Activation of G6PD -> results in increased production of NADPH, increased regeneration of GSH and adequately supported fatty acid synthesis and oxidative metabolism
• Under conditions of insulin resistance and in people with hypertension, increased insulin levels may casue decreased HMS activity-> Decrease availability of NADPH, failing to recycle GSH, inadequately supporting fatty acid synthesis and increasing oxidative stress
(4) Dysglycemia, Steroid Metabolism and Inflammation
• Hyperinsulinemia is one of the key feedback mechanisms in the body for reducing synthesis of the steroid hormone dehydroepiandrosterone (DHEA) by the body’s adrenal glands
• Under hyperinsulinemic conditions, instead of shuffling the steroid 17-hydroxypregnenolone (17-HP) into DHEA, the adrenals convert 17-HP into cortisol (stress hormone) whose activation is associated with both physical and psychological stress
• Combination of elevated cortisol and depressed DHEA also results in imbalanced activity within the liver’s cytochrome P450 oxidase enzyme system with overstimulation of some components and overinhibition of others which finally results in increased oxidative stress and increased risk of inflammation from this oxidative trigger
The effects of carbohydrates, fats, proteins on dysglycemia indicating how one would educate a client /patient in relevant and practical techniques for promotion and maintenance of optimal health in this area
Carbohydrates [2; pages 11,19]
- Simple monosaccharides can have complex metabolic roles and even structurally, they can have far reaching health consequences e.g. the deposition of galactose in the neuronal myelin sheath and the glycosylation of proteins (now known as a co-translational event)
- A functional understanding of carbohydrates has to consider their biological effects as well as their physical properties; e.g. a fibre might be soluble or insoluble, might resist digestion and act as a prebiotic and might also affect blood sugar control
- Studies have shown that liver cells use fructose without mediating the effects of insulin hence fructose has been suggested as less problematic that glucose in dysglycemic individuals
- Consuming large amounts of fructose (greater than 50g) however has been reported to result in an increase in serum triglycerides in some non-insulin dependent diabetes especially those with hypertriglyceridemia. Also causes hyperuricemia in gout patients. Hence intake should be modest
- Resistant starch has very important clinical implications especially in management of blood sugar and diabetes. It also serves as fermentable substrate or food for bacteria in the lower intestinal tract
- Soluble fibres can delay gastric emptying and increase the satiety value of a meal
- Fibre intake has been shown to be cardioprotective, glucose regulating and cancer ptotective
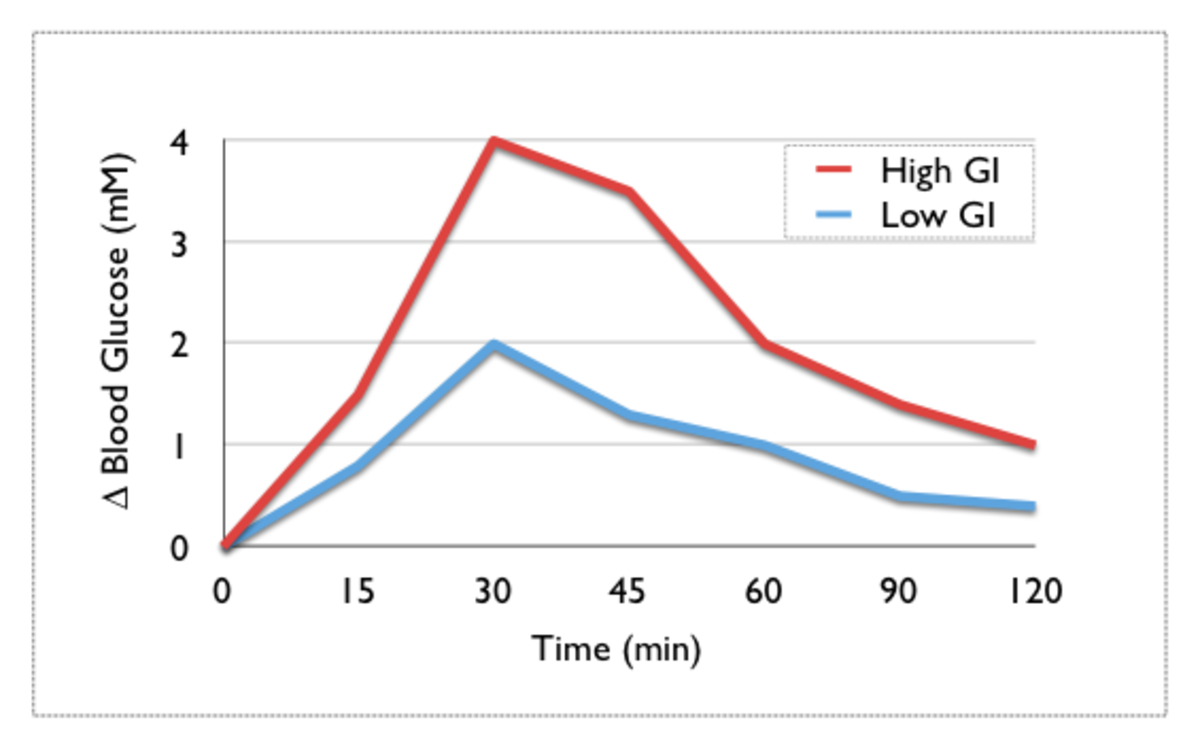
- Carbohydrate metabolism plays an important role in treating both types of diabetes; much research focused on ways to identify high-risk foods for diabetics and using the glycemic index and glycemic load to ascertain the suitability of foods for diabeticsLowglycemic index foods are recommended for people at risk of diabetes and diabetics to allow a more stable blood glucose profile. The concept of glycemic load is more important in the sense that regardless of glycemic index, we need to take account the amount of food consumed respective to its GI value to conclude the final glycemic response obtained rather than rely on glycemic index alone
- . High GI foods and high GL diets produce increased serum glucose levels and increased insulin demand.
Fats [2; pages 69-93]
- The amount of fats consumed can cause high triglyceride levels which can cause increased levels of glucose in the blood as triglycerides increase blood sugar. Of course this is dependant on the types of fats consumed as different fats have different effects. Even though the diabetes association usually recommends one stays away or need to reduce the intake of saturated fats, not all saturated fats are the same especially since short-chain fatty acids (like butyric acid which is highly concentrated in butter) play a very critical role in supporting the health of the intestinal cell lining.
- DHEA has been shown to have anti-diabetogenic, anti-stress and weight-loss promoting effects. Levels of DHEA is decreased with emotional stress and increased in post-menopausal women with breast cancer
- Simply reducing dietary fat intake may not necessarily result in improved health status. Instead, we need to recognize the roles that fats have in structure and physiologic functioning before establishing the appropriate dietary and supplementary fatty acid intake for individuals
- In Syndrome X individuals, high fat diets with the right types of fats may better support eicosanoid synthesis, avoid insulin hypersecretion and recue dyslipidemia and insulin resistance
Proteins [2]
- Individuals may overproduce homocysteine due to metabolic imbalances in cobalamine or folate status or due to an inborn deficiency of sytathioninebetasynthase or methylenetetrahydrofolatereductase (MTHFR)
- Recent research links increased plasma homocysteine levels to an increased risk of heart disease
- Soy protein has been linked with hypocholesterolemic effects
- Soy protein contains adequate quantities of the essential amino acids histidine, isoleucine, leucine, lysine, tryptophan, valine, phenylalanine and tyrosine
- Clinical trials concluded that the consumption of soy protein rather than animal protein significantly decreased serum concentrations of total cholesterol, LDL cholesterol and triglycerides in hypercholesterolemic individuals
- The 2 best supported theories suggest that the type and/or amino acid composition of the soy protein and the isoflavones are key in lowering cholesterol
- Diets low in saturated fat and cholesterol that include 25 grams a day of soy protein may reduce the risk of heart disease
- High levels of triglycerides can elevate blood sugar and cause dyslipidemia
Factors affecting glycemic response [2; page 34-35]
- Carbohydrates higher in amylose have a lower GI as starch amylose is digested more slowly than amylopectin because amylose can form resistant starch and is therefore less digestible and healthier
- Add fibre (50% soluble, 50% insoluble) to diet improves glycemic control (e.g. barley and oatmeal due to high content of fibre beta-glucan in their respective grains)
- Phytate is foods reduce digestibility of foods and hence lowers the GI
- Carbohydrates are absorbed more slowly with increased meal frequency thus resulting in reduction of insulin response, postprandial blood glucose and serum cholesterol levels
- Eat fresh whole unprocessed foods as the ripeness of fruit for example (e.g. banana) can change its GI value
What minerals are considered essential in the treatment of sugar variations? [2;page 169 onwards]
- Chromium:
- Is a major component in glucose tolerance factor (GTF) along with niacin (Vitamin B3) and the amino acids glycine, glutamic acid and cysteine
- Chromium has a strong insulin-enhancing activity. It can help bind insulin to its receptors in the cellular membrane
- Chromium may have an effect on lipid metabolism as well and increase HDL levels (the good cholesterol)
- Improve immune response as it may decrease serum cortisol and increase immunoglobulins
- Selenium:
- can be toxic in amounts greater than 900mcg per day
- is considered an essential mineral
- Is beneficial in individuals whose histories indicate recurrent infections(as in the case if uncontrolled diabetes or dysglycemia), difficulties controlling inflammatory disorders, fatigue or other indicators of oxidative stress or family history of cancer
- Manganese:
- Helps with carbohydrate metabolism, bone development, prothrombin synthesis, protein digestion, collagen formation, fatty acid synthesis and protein synthesis
- Is a cofactor in a number of enzymes important in energy production and antioxidant defense (e.g. superoxide dismustase)
- Copper
- Copper deficiency may result in iron deficiency anaemia
- Copper deficiency may result in poor collagen integrity (as revealed by breaking of blood vessels) and bone and joint problems as well as lipid problems
- Low copper status is also associated with reduced skin pigmentation, central nervous system impairment and osteoporosis
- Insufficient copper limits its role in energy production and enzymatic reactions
- Zinc
- Is important in the functioning of many enzymes and assists with many hormone activities (thymic hormones, growth hormones and insulin) and thus essential in immune function
- A cofactor in a number of enzymatic reactions
- Important for protein and DNA synthesis, wound healing, bone structure, immune function and skin oil gland function. Also important for healthy prostate tissue
- Vitamin B3
- Structure
- Includes Nicotinic Acid and its nicotinamide derivatives
- Niacin is used to form the active coenzymes/cofactors NADH and NADPH which are important in many oxidation-reduction reactions in the body especially in those involved with energy
- Absorption
- Absorption occurs in stomach and intestines by both sodium-dependent facilitated diffusion (at lower concentrations) and passive diffusion
- The NADH and NADPH forms represent dietary Niacin which is hydrolysed for absorption
- Synthesis of Vitamin B3 occurs from tryptophan with Vitamin B6, Riboflavin and Iron as cofactors
- The conversion of extracellular nicotinamide into NADH seems to be under hepatic control and regulated hormonally
- The liver stores excess plasma nicotinamide as unbound NAD
- The nicotinamide that is formed from this NADH degradation can be converted into NADH in most tissues or by microflora in the intestine
- Functional Activity
- The body uses NADH as an electron acceptor or hydrogen donor in many redox reactions
- Also involved in dehydrogenase reactions e.g. conversion of alpha-ketoglutarate to succinate
- NADH is an important cofactor in nonredox reactions
- Glucose Tolerance Factor that plays an important role in insulin response requires Niacin (nicotinic acid)
- Sources [1]
- -Food: torula yeast, brewer’s yeast, rice bran, wheat bran and peanuts
- -Sources of tryptophan: milk, soy, peanuts, eggs, pork, lamb and beef
- Therapeutic Considerations [1]
- -Deficiency causes pellagra (signs include dermatitis, dementia, diarrhoea and death)
- -Niacin is used in:
- *Rheumatoid arthritis and osteoarthritis
- *Diabetes
- *Memory Impairment
- *Intermittent claucidation
- *Depression
- *Lowers LDL cholesterol, lipoprotein A, triglyceride and fibrinogen levels while raising HDL levels
- *therapeutic doses range from 50 to 200mg per day
- Safety and Toxicity [1]
- With as little as 25mg of Niacin, Uncomfortable flushing of the skin may occur but some can tolerate higher levels. Time released niacin has been used to avoid this flushing but this form has been associated with hepatic complications
- Oral administration of as much as 6g/day has been taken without side effects
- Use inositol hexaniacinate instead of niacin to eliminate some of the niacin-associated side effects
- Functional Medicine Considerations [1]
- If patient is using the drug isoniazid, it will compete with the Vitamin B6 needed for tryptophan metabolism to niacin
- If patient has been using high levels of niacin, toxicity may be detected by increased liver enzymes
- With signs of niacin insufficiency, assess if Vitamin B6, Riboflavin and Iron might be deficient too as these may be the underlying cause of niacin insufficiency
Other useful supplements: Vitamins B (for energy production), C, E, A, Cinnamon
How is glycemic balance measured? What problems are encountered in evaluating glycemic balance by the usual methods?
Some methods: Blood sugar, HbA1c levels, Urine, Ketones, Glycemic Index, Glycemic load
Glycemic Index
Carbohydrate metabolism plays an important role in treating both types of diabetes. Much research have been focussed on ways to identify the high-risk foods to avoid or limit in diabetics. This led to the development of the concept of ‘glycemic index. The glycemic index (GI) is aimed to compare different foods based on their ability to induce a rise in blood glucose. It is measured as the value of the blood glucose response to food in comparison to a standard food (usually glucose or white bread). Thus the GI of foods help serve as a guideline to modify the foods we eat to better control blood sugar and insulin levels in both healthy and diabetic individuals.
Glycemic Load
Due to the varied amounts of carbohydrates present in a typical serving of food, a new measure known as the glycemic load (GL) was introduced. The dietary GL is defined as the product of a food’s GI and its carbohydrate content [2]. GL takes into account that foods rated solely on the basis of their GI do not quantify common servings that are eaten [2]. For example, although carrots have a high GI of 92, a usual serving of carrots has a low total carbohydrate content of 6 to 8 grams and therefore should produce a low-glycemic response. Recent research strongly suggest than high GI foods and high GL diets cause increased serum glucose levels and increased insulin demand [2]. These have been shown to increase insulin resistance and the risk of type 2 diabetes in predisposed people.
A prerequisite for looking at glycemic control is the ability to monitor retrospectively long-term glycemic control, specifically, measuring the level of glycated proteins or hemoglobins (HbA1c). Other methods employed in measuring glycemic balance:
- Diabetes self-tests
- *utilises chemically treated plastic glucose testing strips where one compares the colour of the strip to a colour chart which lists various glucose levels
- *Also blood glucose monitors that measures glucose level
- Problems encountered: needs to have accurate calibrated machines
- Fasting blood glucose levels measured six to eight hours after eating;
- Problems: Results may vary based upon what they ate and other predisposing factors
- *Less than 109mg/dL: Normal
- *110-125mg/dL: Fasting blood glucose/borderline, prediabetes
- *126mg/dL and over: Diabetes
- -If tested at random, a number of 200mg/dL or higher is considered diabetes when coupled with other diabetes signs like excessive thirst, unplanned weight loss or fatigue
- Test that is used normally as a diabetic management tool rather than a screening test; more accurate
- Hemoglobin A1c lab test; based on the notion that sugar is sticky
- HbA1c levels:
- 6 = 135mg/dL
- 7 = 170mg/dL
- 8 = 205mg/dL
- 9 = 240mg/dL
- 10 = 275mg/dL
- 11 = 310mg/dL
- 12 = 345mg/dL
REFERENCES
1) Health Schools Australia Course Notes;Part Two; An integrated clinical approach to chronic inflammation; pages 73-98
2) Clinical Nutrition-A functional approach Second Edition, The institute of functional medicine 2004