What is Cancer - A Failure of Cell Death (Chemotherapy)
How do we Fight Cancer?
It seems that I have captured your interest enough to return for the final part in this mini-series (although not the final part in my cancer hubs), If you have hub-hopped to this page and haven't read my previous posts about cancer, then I recommend you nip back and give them a read.
This hub will investigate why cancer therapies have side-effects, some general pathways for efficacy and introduces three types of anti cancer drug. For expediency, they will only be given a brief overview. The interested reader is directed to the links at the bottom of the page and to the upcoming hub mini series on Cancer Therapies.
Side Effects of Chemotherapy
Treatment of infectious diseases caused by pathogens are selective. By altering pathogenic proteins that have no human (or even eukaryotic) counterpart, the therapy acts as a smart-bomb: the drug seeks out the pathogen, kills it, and leaves the human (relatively) unscathed. This is not the case in cancer treatment: the targets of traditional chemotherapies are often present in both healthy and unhealthy cells. In order to kill the cancer, you must poison the patient.
The aim is to activate apoptosis (as this has none of the pain, swelling, inflammation and susceptibility to infection of necrosis) without killing the patient.Whilst current chemotherapies activate apoptosis, it is often only as a secondary effect of their cytotoxic effects. (Large doses of a poison will cause necrosis, but constant, sub-lethal doses can activate apoptosis...effectively annoying the cell to death). As stated above, chemotherapies often target any proliferating cell (such as those of the hair follicle, stomach and intestinal lining, skin - this is why side-effects tend to localise to these areas). Any rapidly dividing cell in your body is targetted by standard chemotherapies, killing these cells as a form of 'collateral damage.' This leads to stomach pains, hair loss, skin sores and rashes, upset stomachs, etc as the cells controlling these areas are being damaged and destroyed.
Combination Name
| Constituent Drugs
| Treatment Usage
|
|---|---|---|
ABVD
| Doxorubicin (ND) Bleomycin (N) Vinblastine (N) Dacarbazine
| Hodgkin's Lymphoma
|
GemTaxol
| Gemcitabine Paclitaxel (N)
| Breast Cancer
|
MVAC
| Methotrexate Vinblastine (N) Doxorubicin (ND) Cisplatin
| Bladder Cancer
|
FEC-T
| Fluorouracil Epirubicin (ND) Cyclophosphamide Docetaxel (ND)
| Breast cancer
|
CHOP
| Cyclophosphamide Doxorubicin (ND) Vincristine (N) Prednisolone (St)
| Non-Hodgkin's Lymphoma
|
A sample list of combination therapies in common usage against different cancer types
(St) = Steroid (N) = Natural Product (ND) = Natural Product Derivative
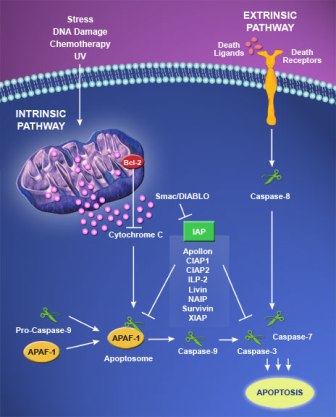
Intrinsic vs Extrinsic Apoptosis
What we Need is a Silver Bullet...
New research is indicating that cancer cells exhibit a higher than normal number of pro-apoptotic receptors that activate the extrinsic pathway. This pathway would activate a series of biomolecules known as caspases from the outside of the cell. Caspases are the cell's executioners. They bring about the required and controlled processes of Apoptosis.
If this research bears fruit, we could soon have a class of drugs that are at least more selective towards cancer cells. This extrinsic pathway is expressed in a wide variety of cancers and, being extrinsic, is independent of the gene p53. This means that even mutations in this gene that tend to render the resulting cancer as aggressive and resistant to many forms of therapy, would not stand in the way of such a treatment.
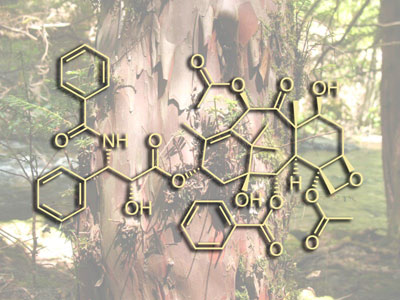
Current Cancer Therapies
- Taxol. Marketed across the world as Paclitaxel, this taxane is one of the most widely used drugs in the war against cancer. Arguably the most important advance in chemotherapy in the past 20 years, Paclitaxel (Taxol) and Docetacel (Taxotere) both inhibit cell division by preventing microtubules from breaking down. Without this controlled disintergration of these 'cellular tow cables' (they are responsible for pulling sister chromatids apart during mitosis), the cell becomes halted at the Metaphase/Anaphase boundary. This ultimately leads to death by intrinsic apoptosis. Often thwarted by acquired drug resistance, but have a broad anti-tumour spectrum
- Gemcitabine - a DNA intercalating agent that promotes cell-cycle arrest by damaging DNA. It does this by competing with nucleosides during DNA synthesis and replication. This is often used in combination with taxanes, such as in breast cancer treatment
- Elemene - derived from Myrrh (yes the gift given to Jesus) Commiphora molmol . Thought to arrest the cell cycle through inhibition of Bcl-2 expression, although this remains unclear. A drug in its infancy, but the object of much attention, particularly in the Far East, where resins containing elemene have been used for medicinal purposes for centuries.
NB - this list is by no means exhaustive...it illustrates three compounds I have experimented with.
What is the Future of Cancer Treatment?
More than 293,000 new cases of cancer (excluding non-melanoma skin cancer) are diagnosed each year in the UK, and more than 1 in 3 people will develop a form of cancer in their lifetime
Cancer therapy is now moving away from mono-therapy to combination therapy, thereby attacking cancers on multiple fronts such as the induction of apoptosis and inhibition of angiogenesis.There is now the search for the holy grail of combination therapy - synergistic interaction - a rare and elusive beast. With phytomedicine and combination therapies, and the advent of the "-omic" technologies, we could well see personalised therapy based on the genetics of an individual's cancer, rather than a one size fits most approach. Nature remains a ready and unrivalled source of inspiration, if not outright agents, for the fight against cancer.
Furthermore, as the field of oncology accepts the heterotypic view of tumours (shown in the first part of this series) and moves away from a reductionist view, novel and inventive therapies are opening up. You needn't slay an entire population of cells, for example, if you can cut off their food source, or convince a critical mass to mutiny...or even commit suicide. The future is looking bright
Where Next? Cancer
- Caspase Cascade & Apoptotic Signals - BioOncology
Learn about the intrinsic apoptotic pathway and how it is regulated through a balance of activity between pro and anti-apoptotic proteins. Then continue to learn about the extrinsic pathway and the caspase cascade