Benlysta -1st Drug to Treat Lupus
Lupus Foundation

A Drug to Specifically Treat Lupus
November 20, 2008, marked the fiftieth year without any medication to actually treat the disease of Systemic Lupus. Clinical Trials.gov describes systemic lupus erythematosus on their website. On November 18th, the FDA approved the sale of Benlysta (Belimumab) to the public and announced this decision on December 9, 2010. Lupus patients had waited a long time for such a drug. Previously patients had been treated with many other harsh medications to suppress the immune system.
I started taking Benestyl Intravenous medication in December and by my third dose I had less joint pain. The drug regimen is as follows: 1 dose every other week times three, then a dose every four weeks. I will be getting my fourth dose in a little over a week and I have great hopes that I will continue to improve and that I will be able to wean off the Prednisone.
S.L.E. Lupus Foundation: Life Without Lupus
Auto-immune Disease
SLE is an often crippling and potentially fatal autoimmune disease that is nine times more prevalent in women than in men. It is four times more likely to affect African American females than Caucasian females. It is suspected that a genetic predisposition along with environmental factors contribute to the clinical manifestation of SLE, but it is not considered to be an inherited disease.
This positive note reinforces our community’s resolve to increase the pace of scientific discovery and clinical development for lupus patients that is made possible by the active participation of lupus patients in clinical trials,
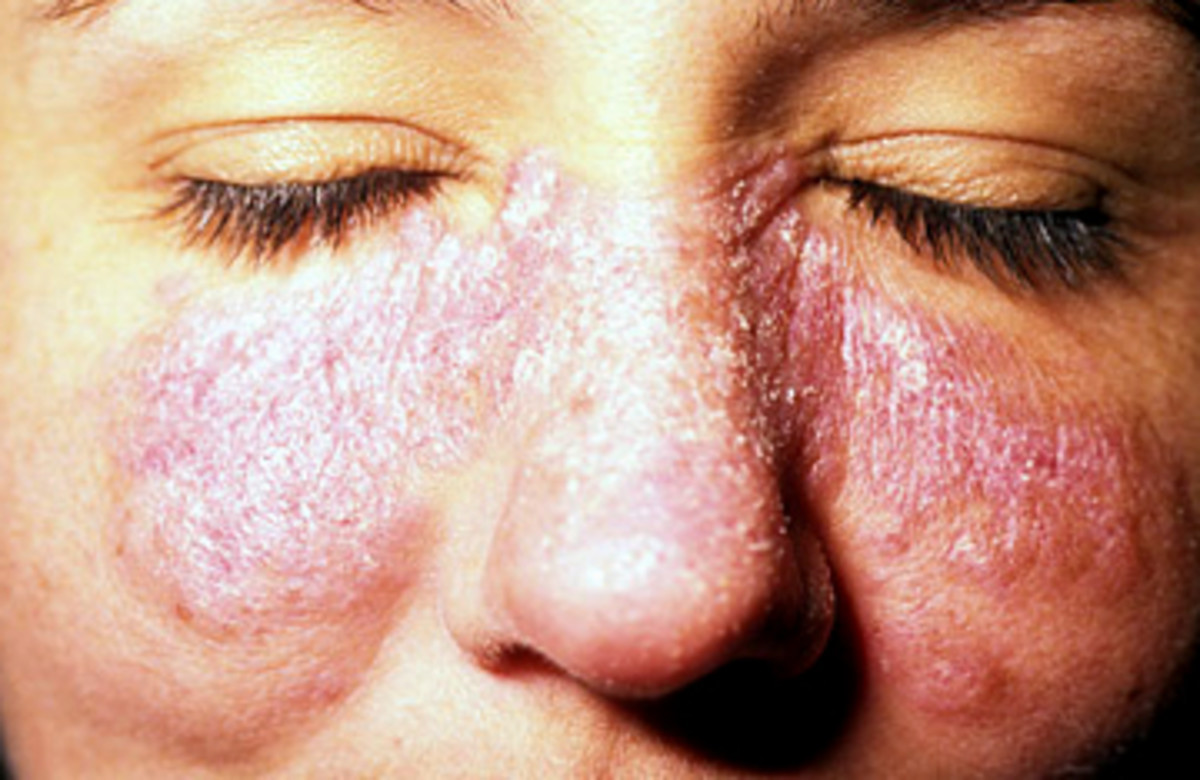
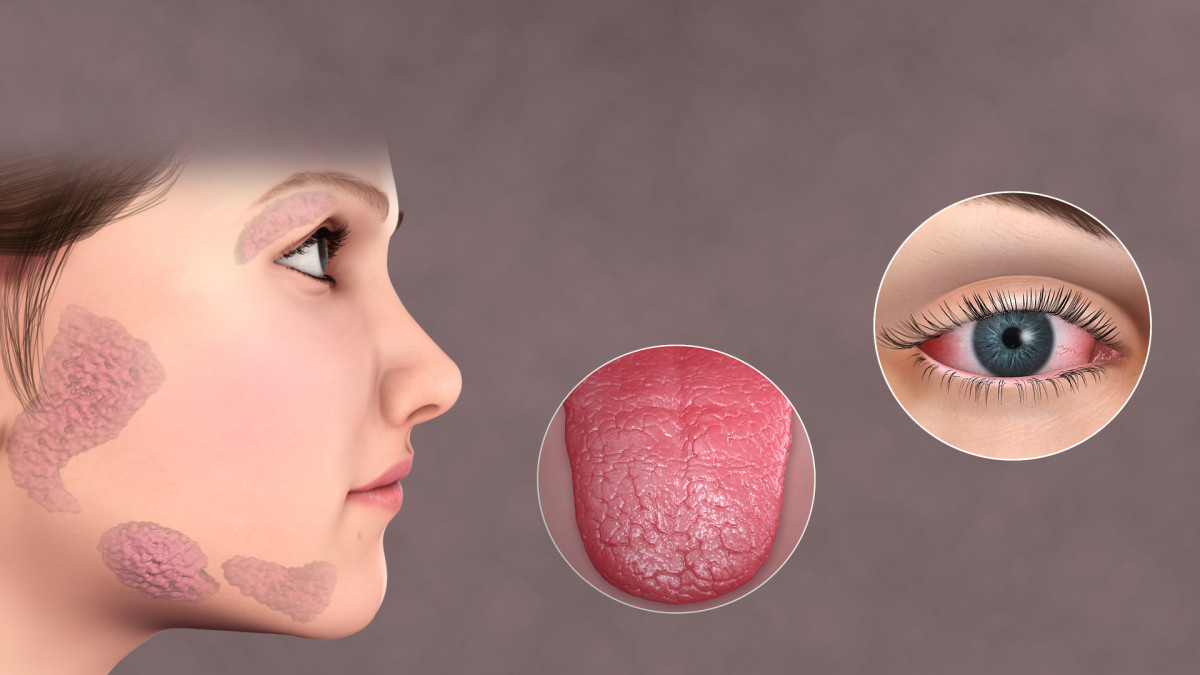
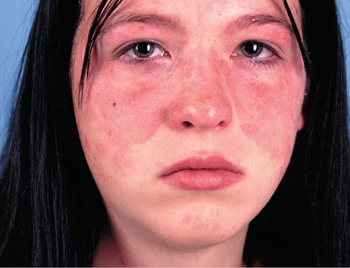
Butterfly Rash
Medical Studies
The new medical studies for Lupus are very exciting as they are actually developing medication that treats the disease rather than just the symptoms.
Medical studies are typically double blind studies so you don’t know if you are receiving the medicine or the placebo. You must also meet the specific criteria for any given study to qualify for their study. Typically these programs accept both genders, patients between 18-70, must have had stable SLE for a duration of at least 6 months and you must sign a written informed consent.
A great deal of lab work is done during the first interview, which includes an ANA screen, HIV test, Hepatitis screen and an EKG. You will be excluded if you have HIV, hepatitis B or C, have had a viral, bacterial or fungal infection within 30 days; have donated blood or experienced a loss of blood >500mL within 4 weeks of randomization or have evidence of renal insufficiency
Other disqualifications: renal glomerular filtration rate <50, total WBC <3000, Neutrophil count <1500, Platelet count <75,000, Hemoglobin <10g/dl, and there is a whole list of other comorbidities that would disqualify you.
FDA Advisory Committee's Recommendation to Approve Benlysta
Lupus Difficult to Diagnose
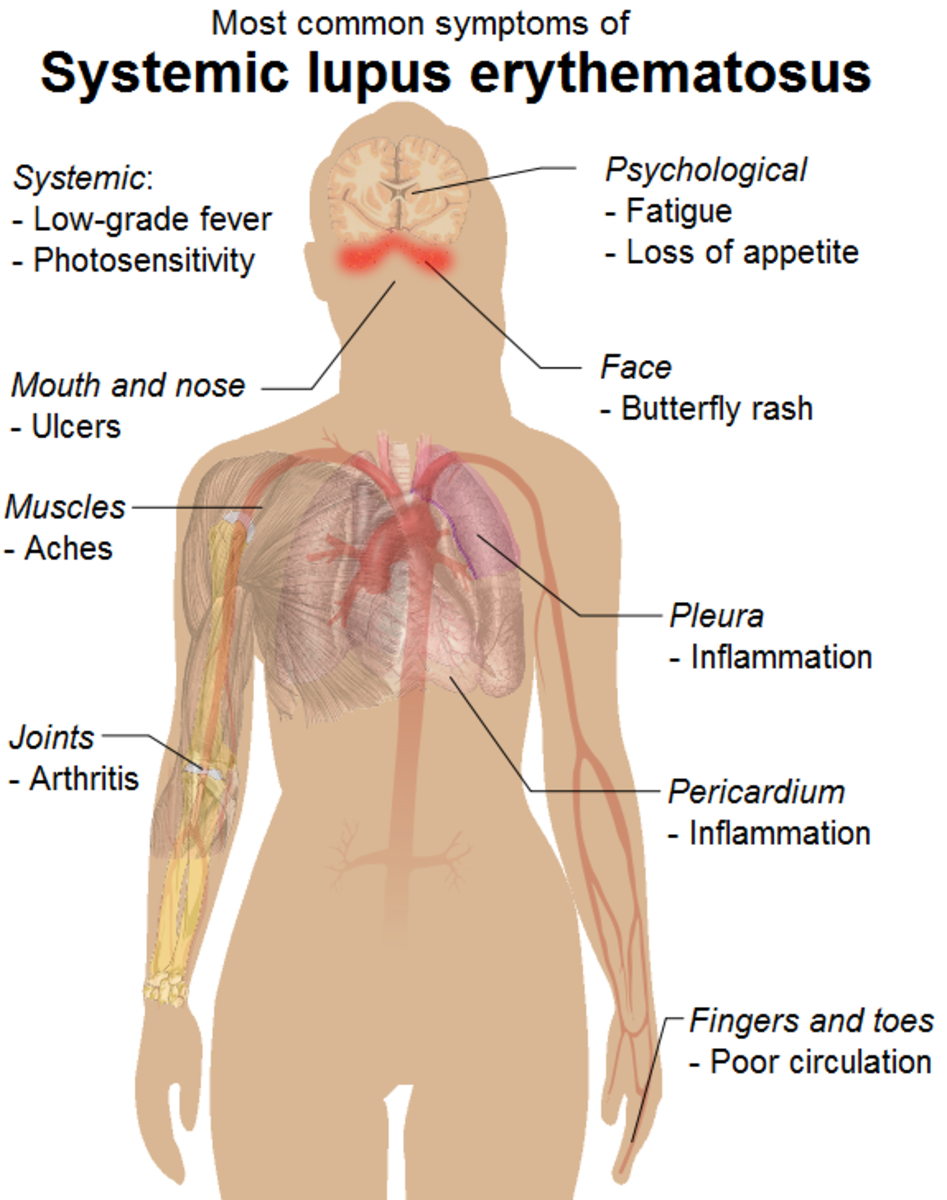
Lupus is typically diagnosed by symptoms and some lab work but there is no specific test for lupus. The more common symptoms are:
- Extreme Fatigue
- Painful and sometimes swollen joints
- Anemia with low RBCs and'or Platelets
- Low grade Fever
- Pleurisy - Pain in the chest when taking a deep breath
- Hair loss
- Rashes, 15% have a butterfly rash which runs across the cheeks and nose
- Sun sensitivity or light sensitivity
- Abnormal blood clotting
- Swelling (edema) in feet, legs, hands and/or around eyes
- Mouth or nose ulcers
- Kidney disease
- Fingers that turn white and/or blue when exposed to cold (Raynaud's syndrome)
In Summary
Benlysta or BLISS-76 has been on the market for over a year. The results have been a Godsend for many lupus patients. I will include their webpage if you would like to read about this medication as the way it functions is a bit too complex for this article.
Belimumab was developed by HGS and GlaxoSmithKline. They completed enrollment for Phase 3 starting in the fall of 2009. The results were again positive, so it will be available on the market in the first part of 2011.
There is a tremendous amount of lupus research happening at the time. The Lupus Foundation of America describes some of the research they are involved with and they have a wealth of information about the disease. If you or a loved one has lupus, please stay informed and learn everything you can to help cope with this disease in your daily life.
This content is accurate and true to the best of the author’s knowledge and does not substitute for diagnosis, prognosis, treatment, prescription, and/or dietary advice from a licensed health professional. Drugs, supplements, and natural remedies may have dangerous side effects. If pregnant or nursing, consult with a qualified provider on an individual basis. Seek immediate help if you are experiencing a medical emergency.