Stress Granules Role in Alzheimer’s and Other Neurodegenerative Diseases: Prevention and Therapy

Many neurodegenerative diseases, such as Alzheimer's (AD), Parkinson’s (PD), and Amyotrophic Lateral Sclerosis (ALS), are characterised by a progressive neuronal dysfunction with cognitive and motor decline. It is well documented that, the deposition of toxic protein aggregates, that occurs inside and outside the neuronal cells, plays a determinant role in the pathogenesis of each of these diseases. In recent years, it has emerged, from different studies, that stress granules (SGs) are involved in the packaging of folded proteins.
The scientists have wondered if stress granules could be the answer to the need for more innovative and effective therapies for neurodegenerative diseases.
Stress Granules: Definition and Function
SGs, as can be understood from their name, are membrane-less cytoplasmic organelles that form transiently in response to stressors. They are very important during hypoxia, that is, a lack of oxygen in the cell, oxidative stress, due to an excess of free radicals, or inflammatory events. In these cases, they have a protective effect in reducing the protein load of the cell and preserving vital resources (Anderson & Kedersha, 2008). When the mechanism of their formation is poorly regulated, they can contribute to the pathological aggregation of misfolded proteins such as those characterising AD, PD, or ALS (Wolozin & Ivanov, 2019).
SGs are dynamic structures composed of mRNA, responsible for the translation of the genetic informations from DNA into proteins, RNA-binding proteins, and other factors involved in translation regulation. They are formed by sequestering untranslated mRNA fragments during cellular stress conditions by a particular physical process that, also explains the reasons for their involvement during pathological conditions. In fact, the mechanism of their formation is atypical and allows the organisation of macromolecules without the use of external membranes surrounding the material being formed. This process is called liquid-liquid phase separation (LLPS). However, this same mechanism can, in vulnerable neuronal cells predispose those to transition toward pathological aggregate states (Banani et al., 2017).

Stress Granules and the Pathogenesis of Alzheimer's Disease
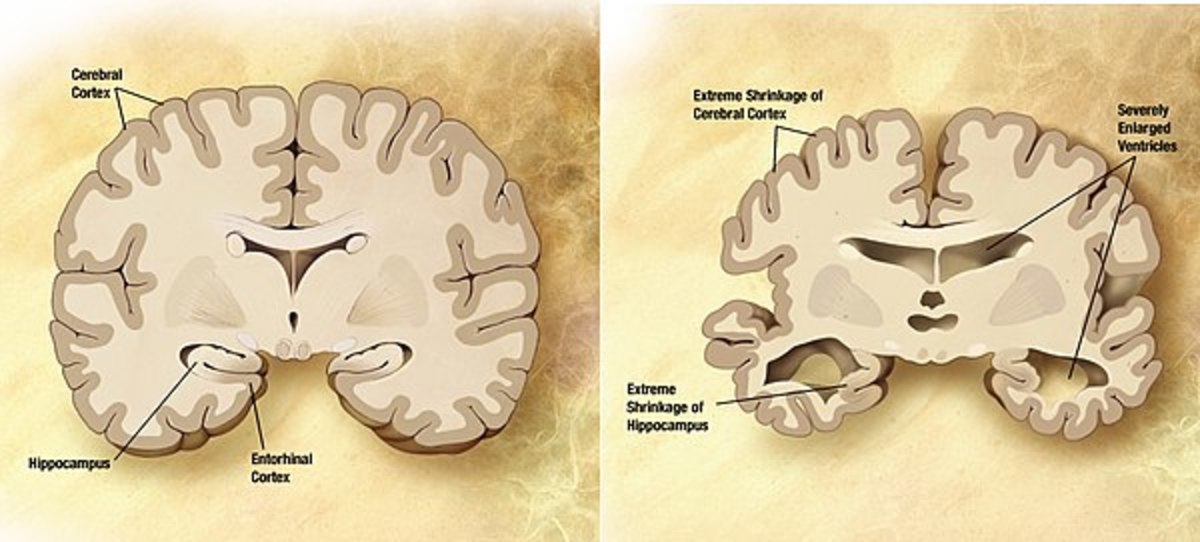
AD is a condition characterised by a slow cognitive and motor decline, with a progressive loss, in affected people, of the ability to perform daily tasks and take care of themselves. Its origin is still not completely clear, and the causes are multifactorial. A pathogenetic hallmark of the disease is the presence of amyloid plaques and neurofibrillar tau tangles within and outside the cells, respectively. The amyloid plaques are constituted by the aggregation of altered beta-amyloid protein, while tau tangles are formed by phosphorylated tau protein.
Recent studies have shown that tau can interact with stress granule components, stabilising them and promoting their transition from a liquid to a solid, aggregated and toxic state (Vanderweyde et al., 2016).
In mouse models, genetic removal of one of the stress granule components reduces tau-associated neurotoxicity and improves memory, suggesting that SGs not only participate in pathology but represent a potential therapeutic target.
Furthermore, chronic stress — a known risk factor for AD — stimulates persistent SGs formation and activates pathways that prevent the proper removal of SGs, such as autophagy, a mechanism by which cells eliminate foreign substances and cellular waste.
Stress Granules and other Neurodegenerative Diseases
SGs also play a crucial role in diseases such as ALS and Parkinson's.
ALS is a neurological disease that affects the brain and the spinal cord. It causes loss of muscle control and can, over time, paralyse muscles needed to speak, breathe, eat, and move, with a fatal end.
The proteins TDP-43 and FUS, which accumulate in aggregate form in many cases of ALS, are normal components of SGs. Mutations in these proteins alter their dynamics, causing them to remain in the granules and promoting neurodegeneration (Ramaswami et al., 2013). In cell and animal models, prolonged induction of SGs leads to synaptic deficits, inflammation and neuronal death, processes shared by many neurodegenerative diseases.
Parkinson’s is a movement disorder, often named, restless paralysis, because the patients are affected by continuous and involuntary tremors in the hands or in other parts of the body. It began slowly, but during its course, it can cause stiffness and unsteadiness in movement, increasing the risk of falls. Psychiatric problems are often associated with anxiety and depression, as well as difficulty in speaking and writing.
In Parkinson’s, oxidative stress is responsible for the mitochondrial dysfunction (mitochondria are considered the energetic centre of the cell) with the consequent accumulation of damaged protein, such as alpha-synuclein. The aggregates of alpha-synuclein passed neuron to neuron and diffused into the brain, propagating the neuronal damage.
In experimental models of Parkinson's, scientists have discovered that the formation of abnormal alpha-synuclein aggregates is facilitated by reduced clearance (a biological definition of the discarding process), leading to their accumulation.
Therapeutic implications
Nowadays, for these devastating conditions, there is no definitive cure. Many therapeutic approaches are promising, from pharmacological classic interventions to monoclonal antibodies. A big difference is represented by early diagnoses now possible, in certain cases, thanks to specific biomarkers of the disease (Vecchio and Colica, 2025). Current evidence suggests that modulating the formation, stability or clearance of SGs may represent an innovative therapeutic strategy. Some approaches under investigation include:
Pharmacological inhibition of SGs' proteic components
Enhancement of autophagy to promote the removal of pathological SGs
Interference with phase separation mechanisms using small molecules
The start-up Aquinnah Pharmaceuticals, co-founded by Benjamin Wolozin, is among the companies developing drugs targeting SG components for the treatment of Alzheimer's disease and ALS. The revolutionary approach has emerged from the studies of Dr Wolozin at Boston University, which have revealed the presence, in AD brain and in experimental models, of tau pathology in SGs. The Aquinnah aims to develop drugs able to disaggregate the tau-SGs complex.
Conclusion
Stress granules represent a point of convergence between cellular stress response, translation regulation and protein pathology. Their involvement in Alzheimer's, ALS and other neurodegenerative conditions makes them a highly promising target for therapeutic research. Understanding and modulating the biology of SGs could open up new avenues for the prevention and treatment of these devastating diseases.
For More Details
- Anderson, P., & Kedersha, N. (2008). Stress granules: the Tao of RNA triage. Trends in Biochemical Sciences, 33(3), 141–150. https://doi.org/10.1016/j.tibs.2007.12.003
- Banani, S. F., et al. (2017). Biomolecular condensates: organisers of cellular biochemistry. Nature Reviews Molecular Cell Biology, 18(5), 285–298. https://doi.org/10.1038/nrm.2017.7
- Ramaswami, M., Taylor, J. P., & Parker, R. (2013). Altered ribostasis: RNA-protein granules in degenerative disorders. Cell, 154(4), 727–736.https://doi.org/10.1016/j.cell.2013.07.038
- Vanderweyde, T., et al. (2016). Interaction of tau with the RNA-binding protein TIA1 regulates tau pathophysiology and toxicity. Cell Reports, 15(7), 1455–1466. https://doi.org/10.1016/j.celrep.2016.04.045
- Vecchio I, Colica C. (2025). New Research on Biomarkers in Alzheimer's Continuum. Rev Recent Clin Trials.https://doi.org/10.2174/0115748871331138250114052615. Epub ahead of print. PMID: 39865820.
- Wolozin, B., & Ivanov, P. (2019). Stress granules and neurodegeneration. Nature Reviews Neuroscience, 20(11), 649–666.https://doi.org/10.1038/s41583-019-0222-5
This content is for informational purposes only and does not substitute for formal and individualized diagnosis, prognosis, treatment, prescription, and/or dietary advice from a licensed medical professional. Do not stop or alter your current course of treatment. If pregnant or nursing, consult with a qualified provider on an individual basis. Seek immediate help if you are experiencing a medical emergency.
© 2025 Immacolata Vecchio