GP Baterije & Rechargable batteries - Where to go next ?
Battery technology and applications
As awkward and unreliable as the early batteries may have been, our descendants may one day look at today’s technology in very much the same way as we now view our predecessors’ clumsy experiments of 100 years ago .
A Look in History
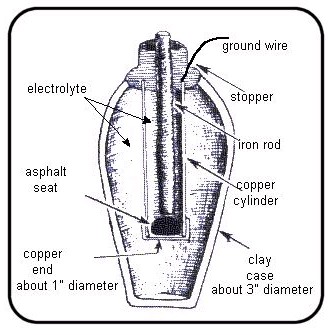
In 1800 Volta discovered that a continuous flow of electrical force was generated when certain fluids were used as ionic conductors to promote anelectrochemical reaction between two metals or electrodes . This led to the invention of the first voltaic cell, better known as a battery.
In 1859 the French physicist Gaston Planté invented the first rechargeable battery. This secondary battery was based on lead–acid (LA) chemistry, a system that is still used today.
In 1899 the Swedish Waldmar Jungner invented the nickel– cadmium (NiCd) battery, based on nickel for the positive and cadmium for the negative electrode. Two years later, Edison came up with an alternative design by replacing cadmium with iron.
Due to high material costs relative to dry cells or LA storage batteries, the practical applications of nickel–cadmium and nickel–iron batteries were limited. In 1932 Schlecht and Ackermann invented the sintered pole plate with which great improvements were achieved. These advancements were reflected in higher load currents and improved longevity.
The sealed nickel– cadmium battery, as we know it today, only became available in 1947, when Neumann succeeded in completely sealing the cell . Soon after the discovery, in the late 1960s, that intermetallic compounds, such as SmCo5 and LaNi5, were able to absorb and also desorb large amounts of hydrogen , it was realized that electrodes made of these materials could serve as a new electrochemical storage medium .

Nickel–cadmium batteries
In the following years the hydride- forming electrode proved to be a serious alternative to the cadmium electrode, which was widely employed in rechargeable nickel–cadmium batteries. In particular, the higher energy storage capacity, good rate capability and non-toxic properties of the chemical elements of which these hydride-forming materials were composed were great advantages in relation to the cadmium electrode .
The nickel–metal hydride (NiMH) and Lithium
The nickel–metal hydride (NiMH) battery became commercially available in the 1990s . The first non-rechargeable lithium batteries appeared in the early 1970s. Attempts to develop rechargeable lithium batteries followed in the 1980s but failed due to safety problems. Because of the inherent instability of lithium metal, especially during charging, research shifted to intercalate lithium ions in host materials in Li-ion batteries. Although lower in energy density than lithium metal, lithium ion is safe, provided certain precautions are taken when charging and discharging, implemented by means of a proper charging algorithm and a safety IC in series with the battery as discussed in the previous chapter. In 1991, the Sony Corporation commercialised the first lithium-ion battery (Li-ion) .
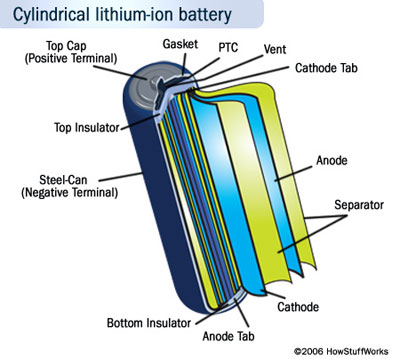
General operational mechanism of batteries
The general operational mechanism of a battery and characteristics of the most important rechargeable batteries available on the market today, e.g. nickel– cadmium, nickel–metal hydride and lithium-ion batteries.
In its simplest definition, a battery is a device capable of converting chemical energy into electrical energy and vice versa. The chemical energy is stored in the electroactive species of the two electrodes inside the battery. The conversions occur through electrochemical reduction-oxidation (redox) or charge-transfer reactions . These reactions involve the exchange of electrons between electroactive species in the two electrodes through an electrical circuit external to the battery.
The reactions take place at the electrode/electrolyte interfaces. When current flows through the battery, an oxidation reaction will take place at the anode and a reduction reaction at the cathode. The oxidation reaction yields electrons to the external circuit, while a reduction reaction takes up these electrons from the external circuit.
The electrolyte serves as an intermediate between the electrodes. It offers a medium for the transfer of ions. Hence, current flow is supported by electrons inside the electrodes and by ions inside the electrolyte. Externally, the current flows through the charger or load . The basic electrochemical unit of a battery is called a cell, but the word battery is commonly used for one cell or for two or more cells connected in series/parallel.
Battery’s lifetime
During a battery’s lifetime, its performance or ‘health’ tends to deteriorate gradually due to irreversible physical and chemical changes that take place with usage and with age until the battery is finally no longer usable. The State-of-Health (SoH) is an indication of the point that has been reached in a battery’s life cycle and a measure of its condition relative to that of a fresh battery.
Aging of the battery
Aging of the battery is a complex process that involves many battery parameters (e.g. impedance, capacity), the most important of which is capacity. If you make a test its clearly shows the discharge capacity (Qd) of a Li-ion battery represented as a function of the cycle number (Cn). The degradation curve has a clearly visible transfer point at which the rate of the battery’s degradation increases.Decrease of the discharge capacity Qd [mAh] as a function of the operational conditions. The exact position of the transfer point varies, depending on the type of battery and operational conditions. Aging of Li-ion batteries is not an absolutely new topic in modern electrochemistry. A number of models describing the aging effects in Li-ion batteries have recently been introduced. Models describing the dynamics of lithium consumption in Li-ion batteries are discussed by Broussely and Spotnitz - Both Broussely and Spotnitz deal with the aging of the batteries under ‘on float’ condition, with the battery being kept under constant voltage of fixed polarity.
A striking feature of all plots in the aforementioned articles is that they are smooth and do not have any transfer points. A first conclusion is that it will be difficult to take into account every nuance of a battery’s charge and discharge qualities and aging characteristics in an SoC indication system.
- Tips on how to save power, be Green, and extend batt...
Review of battery saving apps and solutions for the Motorola Droid and Android 2.x devices. How do you extend battery life of your phone? What is worth trying and what would actually be useful? Find out here.