What is Acidic Soil?

pH
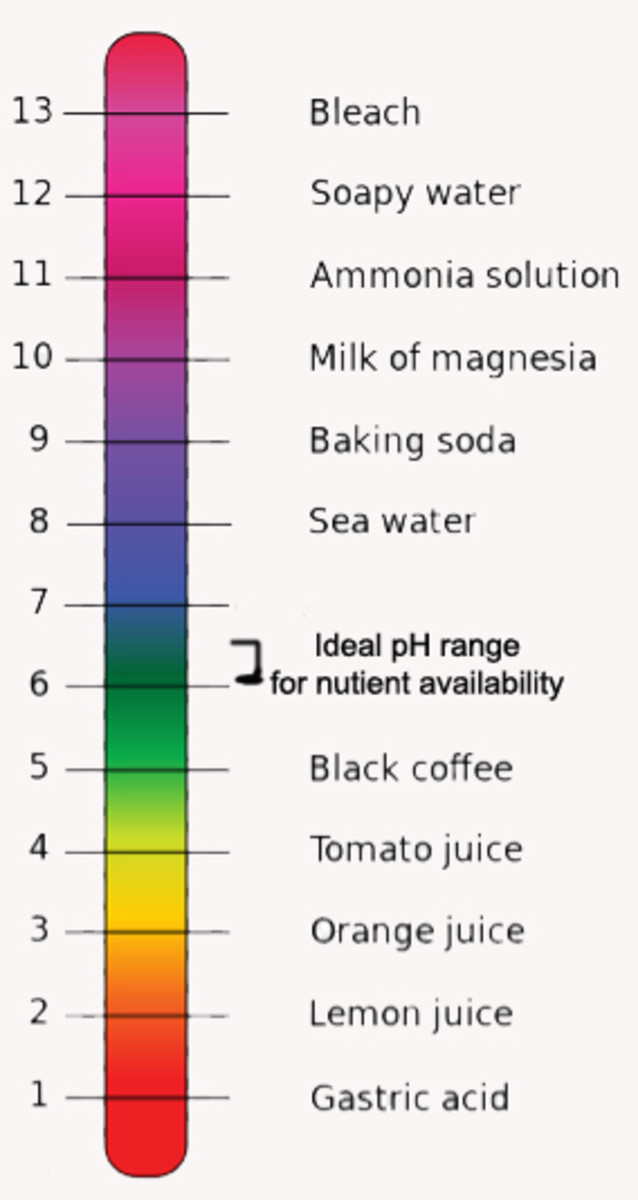
pH is a measurement of a soil that indicates it acidity or alkalinity on a scale of 0 to 14. Soil that is neutral has a pH value of 7.
Acidity
Soil that falls below 7 on the pH scale is considered acidic. It has far more hydrogen ions than hydroxyl ions in its moisture content or soil solution.
Alkalinity
Alkaline soil, which has a pH reading above 7, is sometimes referred to as "sweet" soil.
pH and Acidity
Soil is comprised of three parts: it contains solids, such as minerals, organic matter and microorganisms; liquids, including water and the materials that are dissolved in it; and air which, like our own atmosphere, consists of carbon dioxide, oxygen, nitrogen and other gases.
Soil acidity refers to the potential hydrogen or pH that is found within the soil's liquid portion, also called the soil "solution." Soil pH is measured on a scale of 0 to 14.
Soil that is acidic contains more hydrogen ions than hydroxyl ions, and it falls below 7 on the scale. Soil that is alkaline has a pH level that is above 7 on the scale
Although some plants perform best in acidic soil, most prefer soil that has a neutral pH ranging anywhere from 6.5 to 7.5 on the scale.
Negative Aspects of Acidic Soil
Plants that prefer soil with neutral pH may sicken or even die when planted in soil that is too acidic.
Soil acidity may stunt plant growth.
Acidic soil may contain less calcium and magnesium than most plants require for healthy growth. Additionally, other nutrients that many plants require, such as molybdenum and phosphorous, while present in acidic soil, may be inaccessible to plants.
Acidic soil can prove toxic to some plants.
On the other hand, acidic soil sometimes contains levels of aluminum, copper, iron, manganese and zinc that are unhealthy for the majority of plants. In fact, sometimes the levels can be toxic.
Acidic soil may not have the nitrogen levels that most plants need.
Acidic soil is also an inhospital environment for the bacteria that are part of nitrogen fixation, the process by which nitrogen is turned into a form that plants can use.
How to "Fix" Soil pH
Although a soil's pH level can be altered, there are no quick fixes for either low or high pH. Once amendments are added to the soil, it may take months for the pH level to change.
Organic Matter
Adding compost and other organic matter will ordinarily bring soil to the neutral pH level that the majority of plants prefer.
Lime
If, despite additions of organic matter, the soil pH remains too low (acidic), add lime.
Sulfur
Additions of sulfur will lower the pH of soil that is too sweet (alkaline). Adding a top-dressing of pine needles will also lower the pH level.
ACIDIC SOIL-LOVING PLANTS

Although most plants prefer soil with neutral pH, some popular landscaping plants, such as azalea, rhododendron and hydrangea, thrive in soil that is acidic.
Acid-Loving Plants
| Preferred pH Levels
|
|---|---|
Ash Trees
| 4.5-5.5
|
Azalea
| 4.5-5.5
|
Birch (European white)
| 4.5-6.0
|
Blueberries
| 4.0-5.0
|
Camellia
| 4.5-5.5
|
Holly (English)
| 4.0-5.5
|
Hydrangea
| 4.0-5.0
|
Lily of the Valley
| 4.5-6.0
|
Mountain Laurel
| 4.5-5.5
|
Pine (jack, loblolly, longleaf, white, yellow)
| 4.5-6.0
|
Rhododendron
| 4.5-6.0
|
Spruce (red)
| 4.5-5.0
|