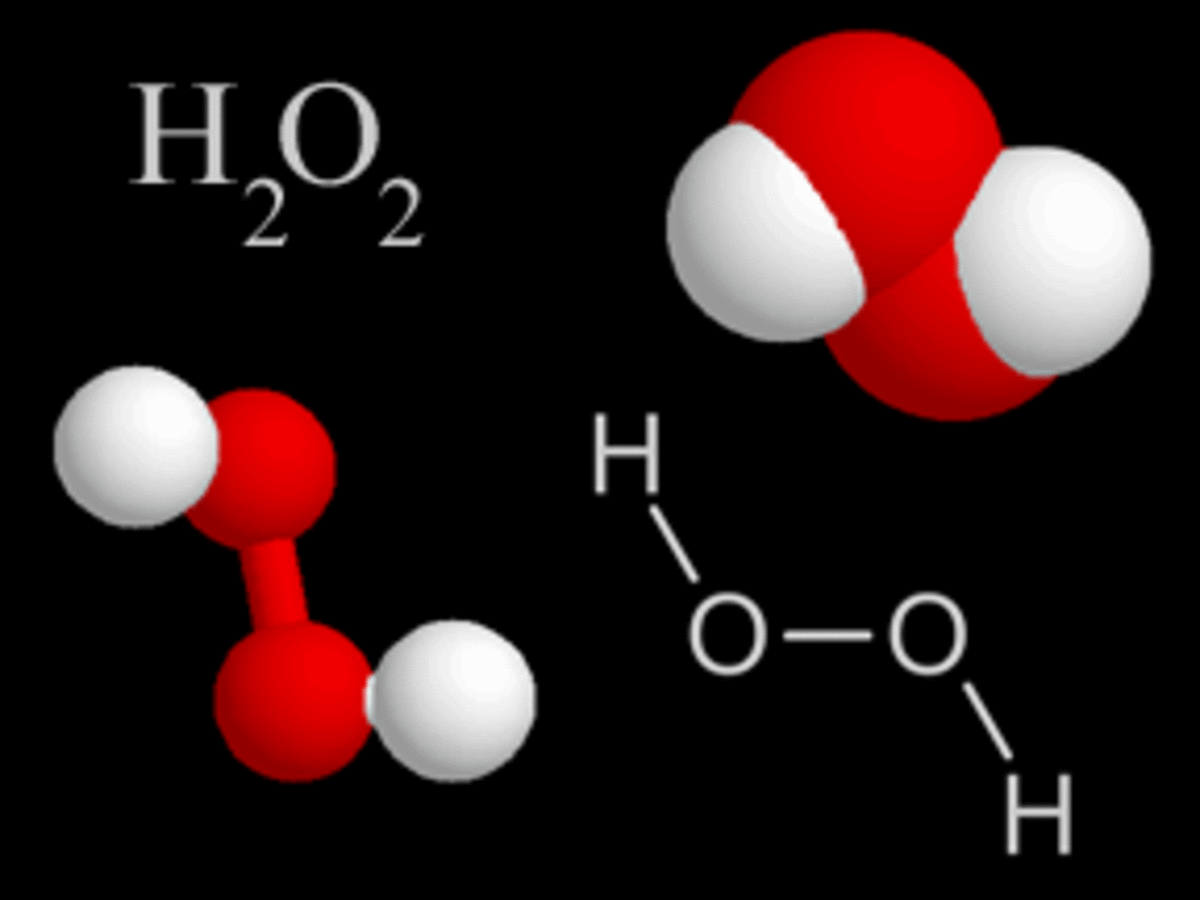
How Does Bleaching Work?
Bleaching is a chemical process for whitening materials. This is done by oxidation or reduction reactions that change the coloring matter to unobjectionable, colorless, insoluble substances or to soluble compounds easily removed by washing.
Commonly bleached products include flour, paper pulp, and some oils. Bleaching processes are most widely used in the textile industry. The chemicals used to bleach textiles are hypochlorites, sulfur dioxide, and hydrogen peroxide. The whitening results from the oxidation and reduction reactions of the chemicals.
Kinds of Bleaches
The most commonly used bleaches are oxidizing agents, which change the coloring matter through reaction with the free oxygen that they liberate. Reducing, or hydro-lyzing, bleaching agents change the impurities of a material by reaction with hydrogen. Oxidizing bleaches include hydrogen peroxide, chlorine, sodium and calcium hypochlorites, sodium chlorite, chlorine dioxide, and potassium permanganate. Reducing bleaches include sulfur dioxide, sulfites, and hydrosulfites.
Home Laundry Bleaches
Home laundry bleaches contain active chlorine or active oxygen as the bleaching agent. Chlorine bleaches may be used on fabrics such as cotton, linen, and, most synthetic fibers; however, they cannot be used on silk, wool, or wash-and-wear fabrics with finishes that retain chlorine. Chlorine bleaches should be diluted before they are added to the washing solution. Clothes should be well ringed after bleaching to prevent fabric damage.
Oxygen home laundry bleaches are usually made of sodium perborate or potassium mono-persulfate. These bleaches are safe for all washable fabrics and finishes, including garments containing spandex fibers. The bleaches based on sodium perborate are most effective when used in hot water and in conjunction with a laundry detergent. Bleaches based on potassium mono-persulfate are intermediate between the chlorine and perborate bleaches in overall bleaching effectiveness.

Cotton and Linen
Before cotton and linen are immersed in bleaching chemicals, such natural impurities as wax and oil must be removed. The cloth is scrubbed and boiled in large kettles, known as kiers. It is then placed for bleachingcin a water solution of sodium hypochlorite or calcium hypochlorite. The hypochlorite ion, or the chlorine formed by the decomposition of hypochlorite ions, oxidizes the natural gray or brown coloring matter of the cloth, changing it into a colorless compound. As a result the textile becomes white.
The rate of bleaching must be carefully controlled by regulating the strength of the hypochlorite solution. Too strong a solution produces too much hypochlorous acid, which will damage the fiber, while too weak a solution does not produce enough chlorine to whiten the cloth properly. The amount of time the cloth is immersed in the solution must also be controlled. Sulfuric acid is used to wash the chemicals out of the cloth before it is dried. Sodium chlorite, and hydrogen peroxide are also used to bleach cotton and linen.
Wool
Before it is bleached, wool is scoured to remove natural oils. Because hypochlorites damage wool, hydrogen peroxide or sulfur dioxide is used instead. In sulfur dioxide bleaching, called stov-ing, the wet wool is placed for about 12 hours in a gas chamber, called a stove. Sulfur is burned in the chamber and forms sulfur dioxide gas. The gas combines by reduction with the coloring matter of the wool to form a colorless compound. The whiteness obtained by this method is brilliant but temporary. Longer-lasting but less brilliant results are obtained when wool is bleached by oxidizing the coloring matter with hydrogen peroxide.
Silk
Silk is scoured before bleaching to remove the sericin, or gum coating. It is then whitened by stoving in the same manner as wool. Hydrogen peroxide is used to bleach only special kinds of silk.
Industrial Bleaching of Textiles
About 95% of all textiles that require whitening during manufacture are bleached with hydrogen peroxide. Cottons can be bleached in continuous processes with dwell-times of as little as 2 minutes. Chlorine-based chemicals used in the past required dwell-times of up to 12 hours.
Continuous bleaching is done in two stages. In the first stage the fabric is saturated with a solution containing caustic soda and trisodium phosphate at temperatures of from 38° to 82° C (100° to 180° F). The cloth then enters a steaming chamber at a temperature of 96° to 100° C (205° to 212° F) for a period of from 2 to 60 minutes, depending on the bleaching requirements. The caustic content of the cloth is then reduced, by washing, to 0.1% or less. In the second stage the cloth is saturated with a bleaching solution containing hydrogen peroxide, sodium silicate, and caustic soda. The cloth then enters another steaming chamber for 2 to 60 minutes. After the steaming, the remaining peroxide solution is removed by washing.
The steaming chambers used in the process can hold from a few hundred to 8,000 pounds of cloth at one time, with the feed into and out of the chamber properly balanced to allow the cloth to remain for the desired amount of time.
Bleaching of Wood Pulp
In bleaching wood pulp, undesirable coloring matter in the pulp is either made soluble or is oxidized to a colorless material by the bleaching process. Chemically cooked pulps (sulfite and kraft) are generally treated with chlorine gas to make soluble the undesirable lignin fraction of the pulp; the pulp is then washed and bleached with sodium or calcium hypochlorite and chlorine dioxide.
Recent developments in kraft pulp bleaching improved the ability of pulp to retain its whiteness, or "brightness," through use of hydrogen peroxide as the final bleaching step. When the peroxide step is used, there is practically no reversion of brightness through succeeding steps of pulp drying and papermaking.
Mechanical pulp or groundwood is frequently bleached by a one-stage hydrogen peroxide or sodium hydrosulfite treatment. In extreme cases, groundwood is bleached by successive treatments of hydrosulfite and peroxide.
Bleaching of Wheat
In the United States, approximately 80% of all wheat flour is bleached. Bleaches permitted by the Food and Drug Administration include oxides of nitrogen, benzoyl peroxide mixed with diluents, chlorine, nitrosyl chloride, and chlorine dioxide. The latter three chemicals not only whiten flour, but also enhance its baking quality by an "artificial aging" action.
This content is accurate and true to the best of the author’s knowledge and is not meant to substitute for formal and individualized advice from a qualified professional.
© 2010 Bits-n-Pieces