Ozone Layer Depletion and the Montreal Protocol
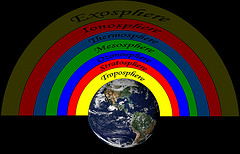
What Is the Stratosphere?
The atmosphere surrounding the earth is made up of several layers. These layers protect the earth and its inhabitants. The troposphere is the layer that we breathe. It goes about as high as the top of Mount Everest.
The next atmospheric layer is the stratosphere. 90% of earth's ozone is located in the stratosphere. Without ozone, humans would cease to exist. The area of the stratosphere that contains the most ozone is called the ozone layer. The ozone layer acts as a protective shield for earth and its life forms.
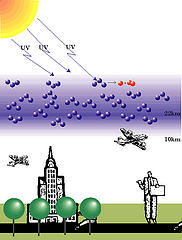
What Is Ozone?
Ozone is almost identical to oxygen. The only difference in ozone and oxygen is that oxygen molecules contain two oxygen atoms. Ozone has three atoms of oxygen. Ozone is so scarce in the atmosphere that there are only about two ozone molecules for every million oxygen molecules.
Ozone blocks the UV radiation by absorbing it. When ozone absorbs the UV rays, the ozone molecule splits. The result is one oxygen molecule and an oxygen atom. This oxygen atom in turn connects itself to another oxygen molecule, again resulting in an ozone molecule. And the process continually repeats itself.
Ozone is essential to human life because it blocks almost 100% of harmful ultraviolet rays entering the earth's atmosphere. Ultraviolet rays are what causes painful sunburns. Too much exposure to ultraviolet light can cause deadly skin cancer and weaken the immune system. Ultraviolet rays can damage plants and marine creatures as well.

CFCs and Ozone Layer Depletion
Manmade materials called fluorocarbons, or CFCs for short, were developed in the 1920s to use as refrigerants. CFCs were viewed as nothing short of spectacular. CFCs were tasteless, odorless, non-flammable, and non-toxic. CFCs were also very stable. These factors made CFCs seem very safe. They replaced earlier refrigerants that were dangerous.
By the 1950s, CFCs were being used in many common products, not just for refrigerants. Any product that could be distributed via a spray can began using CFCs because of the safety factors. Hospitals even used CFCs for sterilization. Deodorant sprays, hair sprays, all types of beauty products, spray paints, and hundreds of other products containing CFCs were being used daily in households around the world. Air conditioning units and, of course, refrigerators contained CFCs as well.
It is estimated that almost 2 million tons of CFCs were released into the earth's atmosphere by the mid 1980s. Because CFCs are so stable, they don't break down easily. This causes them to remain in the atmosphere for a long time. Environmental experts estimate that CFCs remain in the atmosphere for two centuries.
UV rays break down CFC molecules when they enter the stratosphere. When the fluorocarbons are broken down, they release chlorine. Chlorine then binds itself to ozone, which destroys the ozone. Scientists estimate that one chlorine molecule can destroy up to 100,000 ozone molecules.
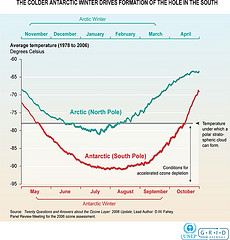
Hole in the Ozone Layer Is Discovered
A so-called hole in the ozone layer was discovered over Antarctica in the mid 1980s. It isn't an actual hole, but the ozone layer is much thinner in this region. A shocking study was published in Nature magazine in 1985. It outlined the depletion of the ozone layer over Antarctica.

What Is the Montreal Protocol?
The ozone layer study caused world governments to take notice. A United Nations agreement called the Montreal Protocol became effective in 1987. This outlined the phasing out and eventual complete elimination of CFCs in modern countries. Studies have shown that the banning of CFCs has already started to result in a thickening of the ozone layer.
It has been estimated that implementing the Montreal Protocol has had several beneficial results. Without the Montreal Protocol, there would be 10 times more ozone depletion than current conditions. This would have resulted in millions of additional cases of skin cancer and other health conditions caused by exposure to ultraviolet sunlight.