Dyes and Dyeing

Dye, a substance, usually colored, which is used to impart color to other materials such as textiles, paper, and plastics. This color must be reasonably permanent (fast) to removal by rubbing, the action of water, soap, solvents, and other detergent agencies; and to chemical or physical alteration through the action of light, heat, gas fumes, or the action of chemicals to which it may normally be subjected. Dyes are usually applied for these purposes by processes of dyeing. Formerly obtained from various natural sources, dyes are now almost entirely produced by chemical synthesis.
Dyeing consists essentially in dissolving a dye, or some chemical modification of a dye or chemicals that can form dyes by interaction, in water or other solvents, and treating the substance to be colored with the solutions. In general, the molecules of the dye are held between the molecules of the material being dyed by physical or by chemical forces, and this is the distinction between dyeing and other forms of coloration. Dyeing (technical) is the uniform coloration of textiles by dyes; printing (textile) is local coloration by essentially the same processes as dyeing, to form single- or multi-colored prints.
Dyeing has been practised for many centuries, as evidenced by the discovery of dyed mummy cloths, etc. Until the middle of the 19th century only a few, natural dyes were available, the majority of which required a mordant for their application. The dyeing process was often laborious and the range of colors limited. Following the discovery of the first successful synthetic dye (mauve) by W. H. Perkin in 1856, a very large number of easily applied, synthetic dyes of many chemical types, covering a much wider color range and often of improved fastness, have been introduced. The production and use of natural dyes has fallen considerably and now represents only a fraction of the total output.
Dyes and dye-forming products are selective in their relations to different kinds of fibers, a given dye being absorbed from solution by some and not by others. When the dye is absorbed it is said to have an 'affinity' or to be 'substantive' to the fiber.
Classification of Dyes
Dyes may be classified either according to the method by which they are applied or according to their chemical constitution. The first method of classification also takes into account the different types of textile fibers. These comprise cellulosic (cotton, flax, viscose, and rayon), cellulose derivatives (cellulose acetate), protein (wool, silk, casein, and groundnut protein), and purely synthetic fibers (nylons, Terylene, and acrylic polymers). For each class of fibers, only a limited range of dyes may be successfully applied.
Dyeing Processes
The method of dyeing depends on the material being treated and on the dye used. To meet modern industrial demands, a speedy process, known as vat dyeing, has been developed. The chemicals used are called vat dyes. The vat dyes are usually applied to a fiber or fabric when it is dry. The fiber or fabric is then put in a reducing solution, which activates the coloring matter of the dye. The dyeing process is continuous and is almost completely mechanized.
Dyes that produce a fully developed color by themselves are called direct dyes. Other dyes require the addition of chemicals to produce the proper color. When a dye cannot attach itself strongly to a fiber or other substance, a chemical, called a fixative, must be used. Fixatives are also used to hasten the speed at which a dye is made to adhere. Dyes may be added to synthetic fibers while the synthetic is still in liquid form.
Basic dyes. The majority of the early synthetic dyes belong to this class, e.g. mauveine and magenta. Other important examples are methylene blue and the rhodamines. They include many dyes which give clear, vivid dyeings of great beauty, but many of them are easily faded by light. Partly for this reason they have now become less popular. They have a natural affinity for protein fibers, to which they are applied from a slightly acid dyebath, the dyes combining with acidic groups in the fiber. They also have an affinity for impure cellulosic fibers such as jute and hemp, but not for pure cellulose (e.g. cotton). A mordant, a substance used to impregnate the fiber and which will unite with the dye, is then required, the most common being tannic acid fixed with tartar emetic.
Acid dyes (e.g. carmoisine, red acid dye). These contain an acidic group and have a natural affinity for protein fibers and nylon, which contain basic groups with which the dyes combine. Applied from an acidic solution, the dyes are subdivided into groups according to the degree of acidity required. Those which are applied from the weakest acid solution have the strongest affinity and have the best fastness to washing and similar treatments. They are known as acid milling dyes. The others, which are applied from stronger acid solutions, are known as levelling dyes, since their poorer fastness permits level dyeings to be readily obtained.
Direct cotton dyes. These are dyes which contain an acidic group and which also have a direct affinity for cellulosic fibers. They do not require the use of a mordant.
Their introduction in 1884 was thus of considerable importance. The first member of the group, Congo red, is a diazo-dye derived from benzidine, and the majority of direct dyes follow this pattern. In application, the addition of a simple salt (sodium chloride or sulphate) is needed to promote affinity and the dyes may be subdivided according to the proportion of salt required. The affinity is also affected by the temperature of the dye-bath and this property is also used to regulate the course of dyeing. Fastness, particularly to washing, is not good, but with some dyes, such as Griesheim red, it may be improved by suitable after-treatments, e.g. with copper or chromium salts, diazotisation, and coupling.
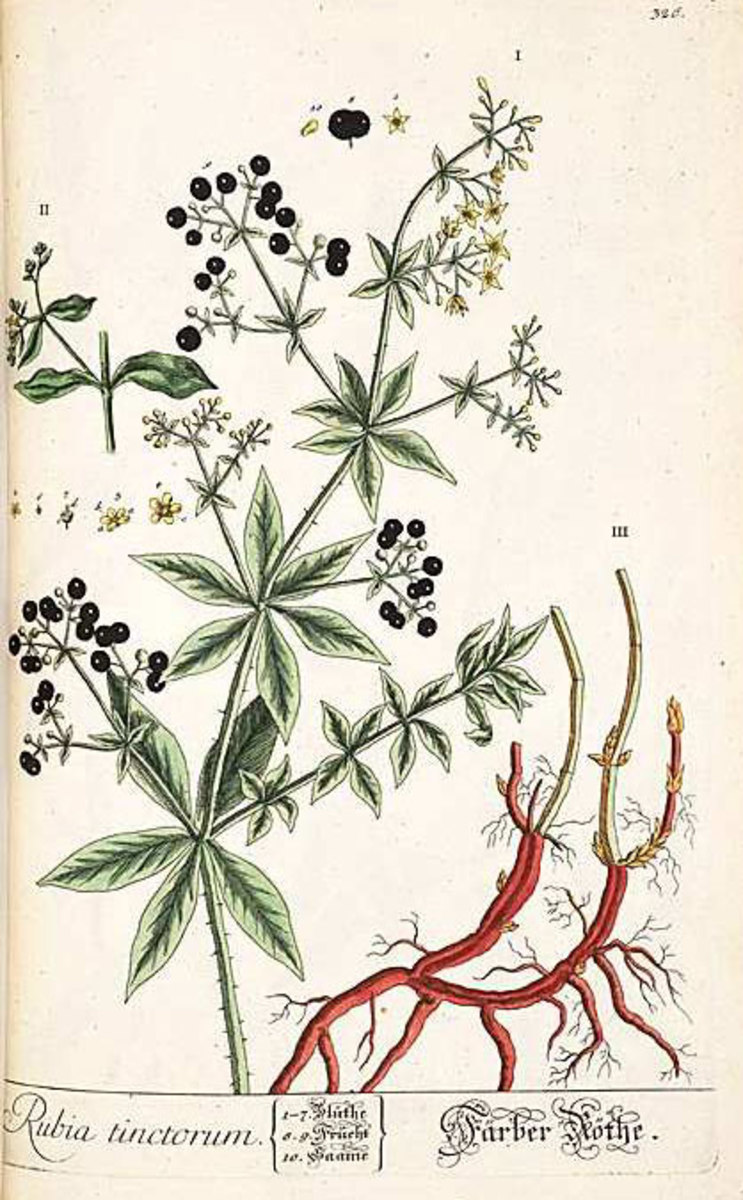
Mordant dyes. This is a class of dyes which are applied with the aid of a second substance (known as the mordant) which is not a dye but which combines with the dye to form a more complex and less soluble molecule, often known as a lake. Mordants are usually metal-containing oxides or hydroxides (e.g. chromium, copper, aluminum, and iron), and these form lakes with essentially acidic dyes. For basic dyes complex acids (e.g. tannic) are used as mordants, but basic dyes are not normally classified as mordant dyes. The majority of the natural coloring matters belong to this group. They may be regarded as true mordant dyes, since they have no affinity unless the mordant is first present on the fiber. This distinguishes them from the modern synthetic mordant dyes which also behave as normal acid dyes, the purpose of the mordant (usually a compound of chromium) being principally to improve the fastness properties. Among important natural mordant dyes is alizarin, obtained from the root of the madder plant but now manufactured synthetically from anthraquinone. Its most important application was on an aluminum mordant to produce the famous Turkey red. On other metal mordants differently colored dyeings are obtained (e.g. tin—pink, iron—violet, chromium-puce). Logwood, obtained from campeachy wood, is the only natural mordant dye still used commercially. Applied on a suitable mordant, e.g. iron or copper, it produces dense black shades. Synthetic mordant dyes (chrome colors) are used for the production of fast dyeing on wool. The mordant (usually a dichromate or chromate) may be applied before, together with, or after the dye, which is itself applied in much the same way as an acid dye.
Metal-containing dyes. These dyes are being used increasingly widely. They represent a logical development from the mordant dyes, the dye-metal complex (usually with chromium or copper) being formed during the manufacturing process rather than on the fiber during dyeing. Dyes of this type are now available both as direct dyes for cellulosic fibers or as neutral dyeing acid dyes for wool and other basic fibers. They have good fastness properties.
Ingrain dyes. The term 'ingrain' is very old and has been used to denote insoluble dyes formed by chemical reaction, on the fiber, of reagents applied either from a
mixed bath or from a succession of baths. The modern technical description places them more specifically into two groups: insoluble azo-dyes and oxidation colors. Insoluble azo dyes are also known as azoic dyes and as ice colors (from the ice employed in preparing the diazonium compound). Conventionally, two components of the dye are applied separately to the fiber. The first is an aromatic hydroxy compound, known as naphthol, and often a phenol, which is applied uniformly in alkaline solution. On subsequently passing the prepared material through a solution of a diazotised amine (a diazo-compound), coupling occurs and results in the precipitation of a colored, insoluble azo-compound inside the fibers. In a refinement of this technique, particularly useful for textile printing, the diazo-compound is converted into a stable, non-coupling form which may safely be mixed with a naphthol without the dye being formed. The two are then applied together. On treating the material with mild acid the diazo-compound is regenerated and coupling again takes place. Since the final dye is insoluble, the fastness to washing, etc., is good. Many members are also resistant to attack during normal processing. Insoluble azo-dyes are used mainly for coloring cellulosic fibers and also, by modified techniques, the newer synthetic fibers. Oxidation colors are black or brown dyes produced by the oxidation of suitable aromatic amines on the fire to be dyed. By far the most important is aniline black, obtained from aniline. Aniline, together with either hydrochloric or sulphuric acid and an oxidizing agent, is first applied to the cloth, which is then heated in a moist atmosphere, and subsequently treated with a dichromate and acid. The aniline is oxidized in a number of stages, resulting finally in the formation of a dense black precipitate within the fiber. Aniline black is applied mainly to cotton, especially by printing, and also gives dense blacks on nylon. Other amines, when oxidised in a similar way, give brown shades and are used for the dyeing of fur and of hair.
Vat dyes. This class includes two natural dyes of great antiquity, indigo and Tyrian purple, but all the rest are synthetic. Their name derives from the ancient practice of fermenting indigo in a vat to render it fit for use. As with the ingrain dyes these dyes are insoluble in water. For application, the insoluble vat dye is first treated with a reducing agent in alkaline solution (usually sodium hydrosulphite in aqueous sodium hydroxide). This is the process of vatting and replaces the older fermentation method. It converts the vat dye into its so-called leuco-compound, which is soluble in the alkaline solution. The material is then dyed with this solution. On exposing the dyed material to the atmosphere, oxidation of the leuco-compound occurs and the insoluble vat dye is re-formed, now within the fiber. Finally, washing and soaping treatments, which remove surplus dye and usually brighten the shade of fixed dye, complete the process. This method is used mainly for cellulosic materials, since the conditions are detrimental to many other fibers. Modified methods of application are often used. Thus, in textile printing, formaldehyde-sulphoxylate is used as the reducing agent. This delays the formation of the leuco-compound until the prints are steamed, this process being an essential one in printing. A different kind of modification is in the preparation, by the manufacturer, of stable, soluble derivatives of the leuco-compounds. These require no chemical treatment by the dyer before use, and may be applied to cellulosic fibers in the same way as direct dyes of low substantivity or to basic fibers as acid dyes. They are reconverted, on the fiber, to the insoluble vat dye by treating the dyed goods with an acidic oxidizing agent, most commonly sodium nitrite in sulphuric acid, but more complex systems are used for printing.
The vat dyes as a class show good all-round fastness properties. The yellow and orange dyes, however, may bring about degradation of the material on which they are dyed if this is exposed for long periods to sunlight.
Sulphur (or sulphide) dyes. These are so called because they all contain sulphur in the molecule and are mostly applied from baths containing sodium sulphide.
Like the vat dyes they are insoluble and are converted into alkali-soluble leuco-compounds for application, in this case generally by the use of sodium sulphide and sodium carbonate. The insoluble dye is regenerated on the fiber by oxidation, usually by the atmosphere. Sulphur dyes do not, as a class, give very bright colors, but mostly represent the cheapest form of intense fast color available for dyeing cotton goods in dark utility shades, black, navy, and khaki.
Disperse dyes. In this class the dyes are also insoluble. Instead of being chemically converted into a water- soluble derivative, the dyes are very finely ground and used in an aqueous dispersion with the assistance of a dispersing agent. They were originally introduced for application to cellulose acetate, which cannot be dyed with the normal water-soluble dyes. More recently they have acquired further importance in dyeing the newer synthetic fibers such as nylon, Terylene, and Orion.
Reactive dyes. These were introduced in 1956. Intended primarily for cellulosic fibers, they contain reac tive groups which allow the dyes to combine chemically with cellulose and thus become an integral part of the fiber molecule. The compounds employed are multinuclear diamines, e.g. Congo red (prepared from bisdiazotised benzidine and naphthionic acid). Salt is added during the dyeing process.
Mass coloration of manufactured fibers. This consists of the inclusion of suitable coloring matters in the fiber solution or molten fiber-substance before'extrusion. The coloring matters are usually insoluble pigments (often azoic, anthraquinonoid or phthalocyanine, but in some cases inorganic) compounded with a dispersing agent. The fibers which result are uniformly colored, and with carefully chosen pigments the fastness properties also are good.
History of Dyes and Dyeing
Natural dyes have been used since ancient times. A garment that was discovered at Thebes, Egypt, and that dates from about 3000 B.C., was dyed with indigo. By 2000 B.C., linen and cloth dyeing had become a fairly common practice. The famous Tyrian purple, obtained from certain mollusks of the Mediterranean Sea, was manufactured by a secret process in the ancient Phoenician city of Tyre. The dye was so expensive that its use was reserved for royalty.
A turning point in the history of dyes came in 1856. In that year an 18-year-old English chemist, named William Henry Perkin, was experimenting in his home laboratory, trying to synthesize the drug quinine. He did not succeed, but he noticed that some of the gummy mass at the bottom of a flask had a purplish glint. When he added alcohol, the mass turned a beautiful purple. Perkin had the substance tested as a dye, and when the tests proved satisfactory he founded a factory to produce the new dye.
Perkin's new dye, named mauve, became very popular. It was the first of the synthetic dyes. Because it was made from aniline, which in turn was made from coal tar, mauve and many similar dyes synthesized thereafter were called aniline dyes or coal-tar dyes. Coal-tar dyes are cheaper, more brilliant, and less likely to fade than any of the natural dyes. Furthermore, methods were found whereby even the natural dyes could be synthesized more cheaply than they could be obtained from nature. By the end of the 19th century synthetic dyes provided a vast number of colors.
When Perkin's teacher, August Wilhelm von Hofmann, returned to his native Germany, he introduced synthetic dyes there. As the country most advanced in chemistry, Germany became, under Hofmann's leadership, the world center for dye manufacture.
During World War I, America's supply of German dyes was cut off, and the American chemical industry was forced to manufacture dyes. As a result the United States became independent of any foreign supply About 80,000 tons of dyes are now manufactured each year in the United States, and more than 3,500 different dyes are used commercially.