Affordable Hydrogen Economy - Getting More from Sunlight
Artificial Photosynthesis
One half hour of sunlight hitting the earth, if harnessed, could supply the planet with all the total energy the entire world currently uses in one year of time.
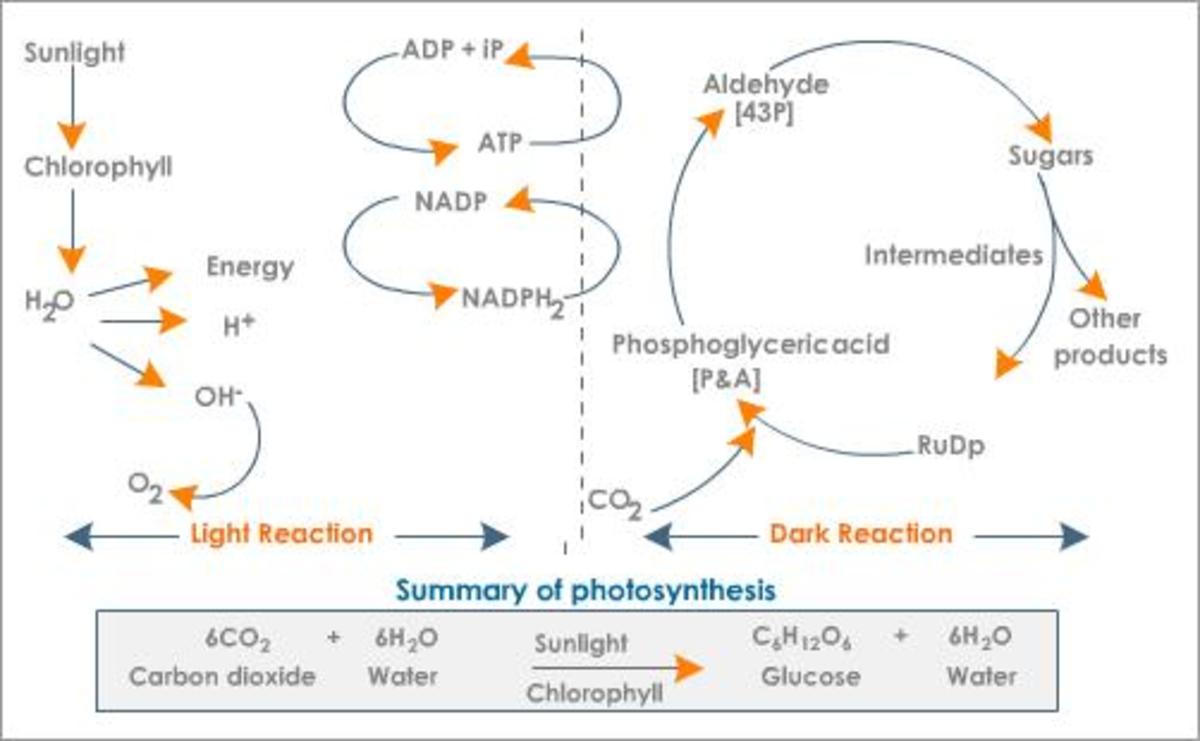
Artificial photosynthesis is a field of research that attempts to duplicate (or closely replicate) the natural process of converting sunlight into energy. This is the process which most (if not all) leafy plants use to convert the energy of sunlight into a more accessible forms of energy that the plant can use. As with most living things energy is stored as simple sugars (carbohydrates).
In the process Carbon Dioxide (CO2) is converted into oxygen and carbohydrates (sugars).
In the past photosynthesis has often referred to the splitting of water into it's constituent atoms oxygen and hydrogen, but as science has delved deeper into the natural process, the term itself has come full-circle, often coming to mean something similar if not exactly the same as the entire process that a green leafy plant goes through to convert sunlight not only into electrical energy, but also sugars. Now artificial photosynthesis, the splitting of water, has come to mean a half-reaction rather than a complete one. The other half is the production of electricity.
A half-reaction, by definition, is the separating water molecules to release hydrogen and oxygen ions. These unstable atoms (ions) are essential in reducing carbon dioxide into a fuel of sorts. Ions are also essential, when used as an energy source, in the production of electricity. Since reducing carbon dioxide into it's constituent ions requires catalysts this hub will concentrate on catalysts which have been found to replicate those found in green leafy plants.
It is important to understand that for artificial photosynthesis to be an effective replacement for fossil fuels, these catalysts must be fast acting, efficient, and long lasting.

Converting Sunlight --- The "Old" Way
There are already photovoltaic cells out there that effectively convert sunlight to electricity. Despite the fact that they are made from silicon, which is an earth-abundant material, they aren't cheap.
Current solar cells, in order to be affordable to the point of competing with hydroelectric or coal, have to be subsidized by local and/or national governments.
They are also fragile, heavy, require expertize to install them, and often require the services of a high voltage (specially trained) technician to connect them to the power grid.
On top of all of that, they convert only a narrow band of total sunlight into energy, often not being able to convert the blue, on the "high" end, or reds and orange bands on the "low" end of the visible light spectrum. This means that most photovoltaic cells made today only convert about a third of the sunlight hitting them. They just aren't as efficient as they could be.
Idealized Photovoltaic Cell
The best photovoltaic cell would be cheaply made and very flexible. Flexible to the point that you could roll it out like plastic wrap.
Such material would not require a heavy glass enclosure to protect it nor would it require a heavy metal frame to mount it in.
The ideal material would also be easy to install and not require specialized skills to install it. In other words a homeowner should be able to install them with a few handtools and no special skills.
Ideally, it would work with all wavelengths of light, not just a narrow band mentioned above. It would also be made of "earth abundant" materials, and last a very long time. Decades if not longer.
The whys of all these requirements are simple.
For photovoltaic cells to replace all fossil-fuel sources of power by 2030, there will have to be one million homes with such cells installed upon them every single day until that date. For a million solar cells to be installed daily for the next twenty years they will have to be something a home hobbiest or DIYer can pull off.
"We don't want to have panels that are treated like glass museum pieces, that are held up delicately on your roof..." - Nathaniel Lewis
That's a tall order.



Nate Lewis & The Lewis Research Group
The Lewis Research Group of California Institute of Technology has been researching catalysts, in particular anodes, that are made of "earth abundant" materials. These materials can be used to convert sunlight directly to electrical energy.
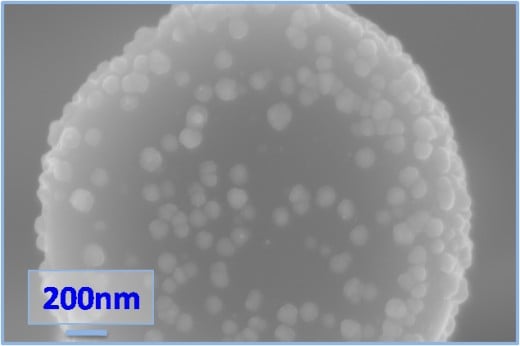
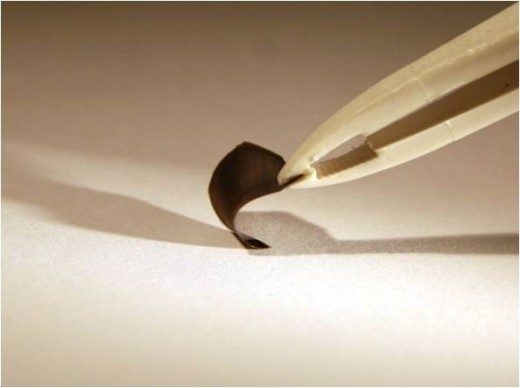
What the Lewis Research Group has been working on is photovoltaics that meet all of the requirements above. So far the group is in the experimental stage, but they have been able to produce prototype films that are covered with silicate nanowires (third photo above right) that respond to all wavelengths of light. These nanowires stand perpendicular to the surface of the film. Because of their length they work with the shortest and longest wavelengths of light and all of the ranges between. This makes them capable of capturing and converting roughly 85% of the energy hitting them rather than 30%.
Voltage is produced along the length of the nanowire. Along the outside of each nanowire is a catalyst that routes that produced voltage radially away from the center of each wire into the polymer substrate and then to the conductor along the edge of the film.
Because noble metal catalysts are out of the question the Lewis Group has been searching for and experimenting with more common elements. Initially the catalyst used on the silicone nanowires was Platinum in small quantities. The white dots in the first image above right is the platinum.
The Lewis group, over time, found that a Nickel Molybdenum alloy worked as well and since these two elements are "earth-abundant" they are much more economically responsible than platinum. In the second image above right the NiMo alloy is seen as the small white dots on the end of the nanowire.
"We have ways to roll this stuff out like carpet that you buy from Home Depot." - Nathaniel Lews
What the Lewis group is most interested in doing is creating a long lasting durable film populated by a "forest" of nanowires that will both produce electricity and/or fuel. The idea is to replicate what a plant does, as close as possible. Lewis feels it might be possible for his nanowire films to not only extract electricity, but also combine carbon dioxide with the hydrogen from water and possibly create a fuel similar to methanol or ethanol.
In other words, create a film that creates electricity and converts fuel from CO2 and water.
Terms and Definitions
Term
| Definition
|
|---|---|
Carbohydrate
| A simple sugar that serves as food source of energy for an animal or plant. Also known as a saccharide
|
Photosynthesis
| The process by which plants create simple sugars and oxygen from carbon dioxide, water, and light energy.
|
Ion
| An atom bearing an electrical charge due to an unequal number of electrons to protons in it's makeup.
|
Cation
| An atom bearing a postitive electrical charge (see ion). e.g. having fewer electrons than protons.
|
Anion
| An atom bearing a negative electtrical charge (see ion) e.g. having more electrons than protons.
|
Coda
Though this hub covers some topics that seem to offer solutions to all or most of the problems presented by solar cells, it doesn't.
The reader should bear in mind that at the time of this writing the solar cell replacements discussed here are in early development and testing stages. They are not yet commercially available and no date or time has been proposed for making them available to contractors or the general public.
And, even if these materials were past that research and development stage and widely available, there are still problems with acceptance and funding. Just because something seems like a good idea does not mean that it will be seen as profitable, desirable, or even necessary.
Global Warming
Many of my readers may have already noticed that I never actually discuss the topic of "global warming."
In truth, whether or not there is indeed a problem of global warming doesn't concern me.
What does concern me is pollution and waste.
No matter how cleanly a car can burn gasoline it still pollutes far more than one that would run on hydrogen or electricity produced from burning hydrogen fuel. The same is true of coal fired power plants.
At this point in time it is economically prohibitive to build plants that can take 100% of the pollutants out of the stream of hot gases coming out of a coal fired plant.
Then there's the simple fact that no matter how much coal or oil there is below the ground, there is only so much of it. It cannot possibly last indefinitely. Sunlight, on the other hand, is certain to last another couple billion years. In fact, another four point five billion at minimum.
And as the top of this article points out, if we can capture enough sunlight and find a way to store it, we'll have more than enough power to meet our every need....and not have to breathe hydrocarbon fumes to do it.
Disclaimer
Though the author stands to make a small profit from this article (hub) and advertising attached to it, the primary focus is to inform readers of advances in solar energy capture.
The author has no control over the ads or the content of those ads attached to this article.