Affordable Hydrogen Economy - Storage
Introduction
Though the title doesn't say as much, the real focus of this article is hydrogen as a fuel for home or automotive purposes. This includes cars, trucks, buses, and other wheeled transport as well as living quarters, apartment houses and even businesses.
Converting sunlight to hydrogen (through the electrolysis of water) is an excellent way of extending solar power beyond sunset.
A Daunting Problem
Generating hydrogen and producing power from it may soon be inexpensive enough that most will be able to replace connection to a power grid, or better still, sell power back to the electric company in the community.
But the biggest problem of all remains storing the generated hydrogen itself for future use. Even if hydrogen can be produced from sunlight and water, cheaply enough that the vast majority of Americans can do it, one of the main reasons to create hydrogen is so that when the sun goes down there is still power enough to light and heat our homes and power our cars.
This means the power must be stored somehow.
Hydrogen can be stored in a variety of different ways. The real problem is in storing it in such a way that it costs less to store it than it does to burn it. This may seem counter-intuitive, but the next section will illustrate this point.

Storing Gaseous Hydrogen under Pressure
The most common way to store this gas is under pressure in a tank designed for that purpose.
The problem with this method comes from compressing the gas and pumping it into a tank. Energy is required to do that and if the energy required exceeds the amount of potential energy from the hydrogen itself, then the process really isn't economical. Part of the problem here is that hydrogen is the lightest and least dense of all the elemental gases. Were it denser more energy could be extracted from it and storing it would be more economical.
Another problem with compressed hydrogen is in its use for automobiles. To get the sort of range most people expect of their vehicles (roughly 300 miles) hydrogen would have to be stored in a vehicle, in a special tank, designed to withstand an internal pressure of 5,000 to 10,000 pounds per square inch (psi). In Europe this would be 350 to 700 bar where one bar is the same as one atmosphere in the U.S.
As one might imagine, this is a lot of pressure. A tank rupture at this sort of pressure, even without a fire or flames, could be quite devastating to the dwelling, vehicle and perhaps the people in either.
So despite the fact that this is the most common way to store hydrogen it's the least cost effective and possibly the most dangerous.
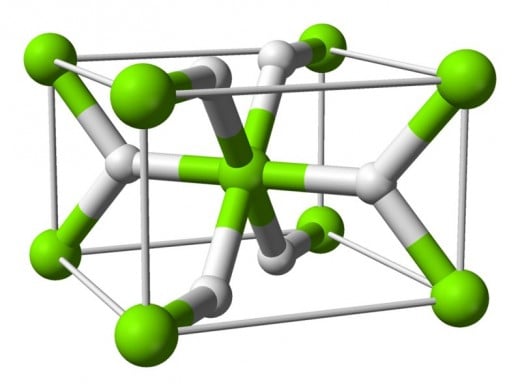
Metal Hydride Storage
Storing hydrogen in a metal matrix has not only been proven it is quite a reliable means of storage.
To date hydrogen has been successfully stored, in very high densities, in Magnesium Hydride, Sodium Aluminum Hydride, Lithium Aluminum Hydride, and Nickel Metal Hydride. The problem isn't the storage capacity so much as the expense of the storage materials themselves. Also, storing hydrogen in any of these matrices involves high pressures and higher than room temperatures to accomplish.
Both the pressures and temperatures required represent an energy expenditure that offsets some of the energy benefit of the hydrogen itself. Then there's the problem of releasing hydrogen for use from these materials which also involves an energy expense. We are talking about temperatures ranging from 245° F to 390° F in both instances.
Storing Refrigerated Liquid Hydrogen
For hydrogen to be stored in a liquid form it must be put under pressure and brought down to a temperature of −423.17 °F (−252.87°C). Of course, at these temperatures, liquid hydrogen not only must be stored under pressure, it must also be stored in a special cryogenic tank insulated from outside temperatures.
Naturally getting hydrogen down to these temperatures comes at a high energy cost. The same cannot be said for getting the hydrogen back up to usable temperature, but a substantial amount of tubing/piping would be required in the way of a heat exchanger to accomplish this.
This represents added weight which is also a problem when considering this means of storage for automotive use.
Storing Hydrogen as a Room Temperature Liquid
Petrochemical Storage
We already do this in the form of gasoline, kerosene, and other fuels. It isn't the hydrogen component of these fuels that makes them especially dirty; it's the other parts of the fuel molecule that makes the burned residues dangers to breathe and ingest.
For that reason there are many alternatives to storing hydrogen as a room temperature fuel other than the petrochemical compounds mentioned above.
Synthetic Hydrocarbon
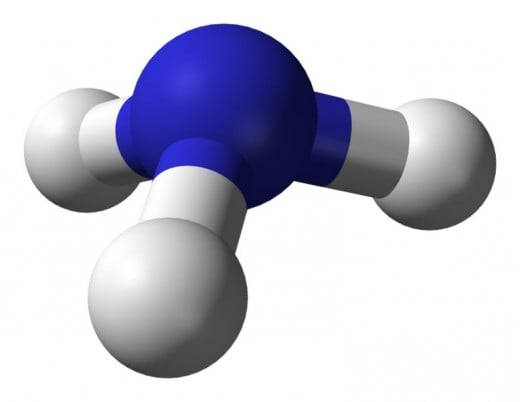
Ammonia: is an excellent medium for storage of hydrogen and though it does not burn readily at atmospheric pressures, it burns quite well in the compression environment of a slightly modified automobile engine.
The downside to ammonia is its toxicity and quite frankly the smell. Ammonia used as fuel must be pure. Finally, because the molecular makeup of ammonia is Nitrogen and Hydrogen there will be nitrous oxides produced during the burning of ammonia.
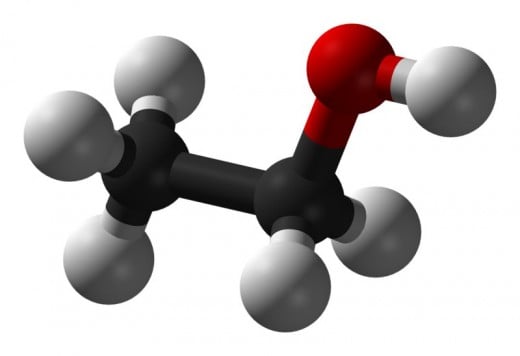
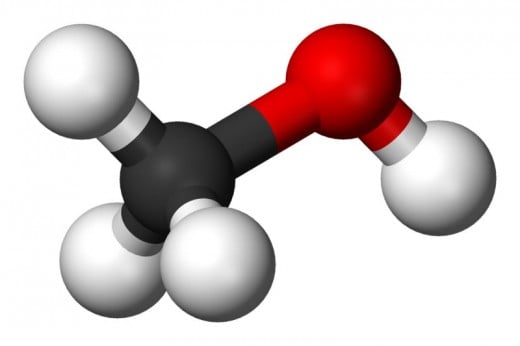
Alcohols: Ethanol and methanol are actually good reservoirs of hydrogen. They can be stored at room temperatures, have relatively high energy density, and are much less polluting than petrochemical hydrocarbons.
Ethanol: The problem with these alcohols is that they are relatively dirty, in some cases, dirtier than petrochemical fuels. In particular ethanol produces formaldehyde. Formaldehyde, in turn, forms ozone when it reacts with sunlight.
Methanol: The problem with this form of alcohol isn't so much its polluting effects as its corrosive effects on metal. In fact any metal that relies on an oxide coating (aluminum, stainless steel, etc.) will suffer corrosive effects from methanol.
Best Storage - For Now
At this point in time the most practical and energy efficient way of storing hydrogen for home and automotive purposes still appears to be in liquid form at atmospheric pressure.
I am hoping that someone comes up with a way of storing this gas, at ambient temperatures, in a metal matrix because this matrix has the highest storage capacity of any of the methods mentioned. Of course, the energy required to unbind the hydrogen from the matrix make current methods impractical and energy expensive.
With all of the other advances in producing hydrogen from sunlight and converting water to its constituent atoms, this seems the last great hurdle.
Disclaimer
The author was not compensated in any way, either monetarily, with discounts, or freebies by any of the companies mentioned.
Though the author does make a small profit for the word count of this article none of that comes directly from the manufacturers mentioned. The author also stands to make a small profit from advertising attached to this article.
The author has no control over either the advertising or the contents of those ads.