Development of the Battery - Better Battery – and Better Battery Life
Static Charge
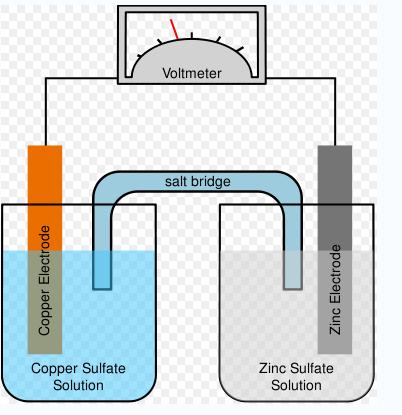
It is estimated that batteries have now been with man for more than 500 years. The earliest documented methods of generating electricity were by generating a static charge. It was not until the year 1800 that a guy called Volta invented a battery called voltaic pile. This voltaic battery was just discs of zinc and copper piled on each other. The discs were separated by cloth soaked in brine. In the year 1836, a British guy by the name Daniel came up with another battery which he named Daniell cell. This Daniel guy was a chemist as well as a meteorologist. Daniel used two pots one with zinc sulfate solution and the other with copper sulfate solution to generate electricity.
Fuel Cell
In 1839, Sir Grove of UK invented the fuel cell where electrons and protons are separated from reactant fuel by a catalyst like in today’s phosphoric-acid fuel cell. In 1859, a Frenchman by the name Planté came up with his lead acid battery which was a rechargeable battery and which people have been using in automobiles even up to today. In 1868, another Frenchman called Georges Leclanche came up with Leclanche cell in which he crushed manganese dioxide, and used a carbon rod as the cathode whilst zinc rod was the anode and were then immersed in a solution of ammonium chloride. In 1888, an American by the name Gassner completed the Leclanche battery into a dry cell which could not leak fluids because he used molten salt – talk about seeing far because this dry cell battery has translated into an industry worth billions of dollars.
Nickel-Cadmium Battery
In 1899, Waldmar Jungner from Sweden came up with the nickel-cadmium battery. The The nickel-cadmium battery was and still is a type of rechargeable battery using nickel oxide hydroxide and metallic cadmium as electrodes. This battery was successfully sealed by a French man by the name Neumann. This sealed battery is the nickel-cadmium we have in use today.
Nickel-Iron Battery
In 1901, Thomas Edison invented the nickel-iron battery which was meant to be storage battery. This battery had a nickel (III) oxide-hydroxide cathode and an iron anode, with an electrolyte of potassium hydroxide. The battery was meant to power electric vehicles. The battery was very robust and was easy to charge. It was however expensive. The nickel-iron battery could be charged continuously thus making it very ideal for backup power supply. The Edison battery company was sold to Exide Battery Corporation who found the battery unprofitable and with no future and discontinued production in 1975.
Union Carbide Corporation
From 1960 onwards the Union Carbide Corporation had developed sufficient financial muscle to carry on the development of batteries for the unending world markets. In particular, they successfully completed the development of alkaline batteries and alkaline cells. An alkaline battery is a battery made of multiple cells. They developed disposable alkaline batteries as well as rechargeable alkaline batteries. The alkaline batteries are batteries dependent upon the reaction between zinc and manganese (IV) oxide.
Nickel-Metal Hydride Cell
In 1989, the nickel-metal hydride cell was successfully developed. The nickel-metal hydride cell is similar to nickel hydrogen cell only that the battery uses a hydrogen-absorbing alloy for the negative electrode instead of cadmium. By 1990, the union carbide corporation jumped into the game they knew playing and successfully commercialized the nickel-metal hydride battery.

Lithium Ion Battery
The lithium battery appeared in 1970 and there was great need to make lithium battery rechargeable. But lithium being a metal, this was very dangerous due to instability of lithium metal. Due to these problems of instability, research was concentrated in using lithium ion which is stable and in 1991, Sony Corporation of Japan was successful in lithium ion battery and it’s in this year that this Sony Corporation commercialized the lithium-ion battery which you and I are today using in your laptop computers and cell mobile phones.
Harvest Solar Power from Outer Space
Today, as you relax with your slim laptop and cell phone, do you ever wonder that it took these guys so many years to come up with a better battery that is presentable and acceptable to you and me? You may be asking yourself why they went looking for better battery life when God had given them enough energy from the sun. The sun has a lot of electricity but one problem has been how to store that energy so that we can use it at night, or when there are clouds or during the winter. If you can see these problems then you can see the logic why you and I should start thinking of how we are to harvest solar power from outer space in the next 500 years. How to get sun energy transmitted from space to the earth is the task we have so that we can avoid the problem of storage.
If you have liked this article, and you would want this page to keep up and improved, you can help by purchasing some great items from Amazon by following Amazon links and widgets on this page. A free way to help would be to link back to this webpage from your web page, blog, or discussion forums.
The Author’s page is designed to help beginners and average readers make some money as an extra income to supplement what they may be earning elsewhere - details of which you can find in My Page, if you will.