How do fuel cells work?
What is a Fuel cell?
Need for efficient and environment friendly energy resources are increasing day by day. People are searching for compact, highly efficient and pollution free alternatives to the exciting conventional and non conventional resources. Fuel cell is one such alternative that can provide highly efficient and pollution free energy at minimum cost.
Fuel cell is a device which produces electricity by combining hydrogen and oxygen. It is the simple, economical and eco friendly form of power generation. There are various types of fuel cells. A basic fuel cell consists of the following parts
- Positive electrode (anode)
- Electrolyte
- Negative electrode (cathode)
The electrolyte is used for the exchange of charged particles from the anode to the cathode. It also acts as the catalyst which speeds up the chemical reactions. Most of the fuel cells make use of hydrogen fuel. Although hydrogen is the basic fuel in the fuel cell, it also requires oxygen.
One of the great advantages of fuel cell is that it is capable of generating power at very less impact to the environment. There are many other advantages also that can be discussed later. A single fuel cell produces very less voltage. Hence several fuel cells are connected in series.
Working
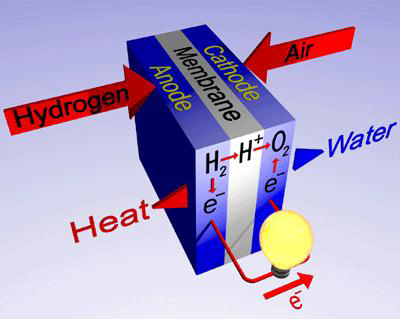
Basic working of fuel cells
There are several types of fuel cells. The workings of each one of them are slightly different from each other. But the basic principle remains the same.
Hydrogen enters the fuel cell through the anode. At the anode the hydrogen molecules react with the catalyst and produces hydrogen ions and electrons. These electrons reach the cathode via an external connection. This flow of electrons constitutes the current flow. The positively charged hydrogen ions reach the cathode through the electrolyte. At the cathode it combines with the supplied oxygen and forms water. This water is exhausted.
Since a single fuel cell could produce very small voltage, number of similar fuel cells are combined together. In order to get an AC supply, the DC output of the fuel cell is fed to the inverter.
Most of the fuel cells are powered using the hydrogen gas. Hydrocarbon fuels such as natural gas, methanol, or gasoline can also be used.
Types
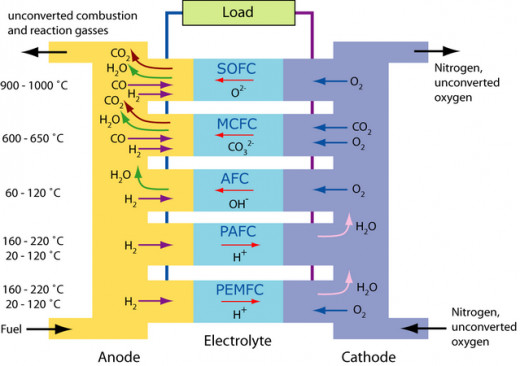
Important types of fuel cells
1. Proton Exchange Membrane Fuel Cell (PEM)
2. Direct Methanol Fuel Cell (DMFC)
3. Alkaline Fuel Cell (AFC)
4. Phosphoric Acid Fuel Cell (PAFC)
5. Molten Carbonate Fuel Cell (MCFC)
6. Solid Oxide Fuel Cells (SOFC)
Type
| Electrolyte
| Catalyst
| Operating Temperature
| Electrical Efficiency
|
|---|---|---|---|---|
Proton Exchange Membrane Fuel Cell (PEM)
| Solid polymer membrane
| Platinum
| 175-200⁰F
| 40-60 percent
|
Direct Methanol Fuel Cell (DMFC)
| Solid polymer membrane
| Platinum
| 125-250⁰F
| 40-60 percent
|
Alkaline Fuel Cell (AFC)
| Potassium hydroxide solution in water
| non-precious metal catalysts
| 225-475⁰F
| 60-70 percent
|
Phosphoric Acid Fuel Cell (PAFC)
| Liquid phosphoric acid ceramic in a lithium aluminum oxide matrix
| Platinum
| 350-400⁰F
| 36-42 percent
|
Molten Carbonate Fuel Cell (MCFC)
| alkali (Na & K) carbonates retained in a ceramic matrix of LiHO2
| non-platinum group catalysts
| Around 1,200 ⁰F
| 50-60 percent
|
Solid Oxide Fuel Cells (SOFC)
| yttria-stabilized zirconia (YSZ)
| non-platinum group catalysts
| About 1,800⁰F
| 50-60 percent
|
Fuel Cell vehicles
The main aim of using fuel cells in vehicles is to reduce environmental pollution. Fuel cells, when powered with pure hydrogen can give an efficiency as high as 80 per cent. If pure hydrogen is not available, a reformer, that turns hydrocarbon or alcohol fuels into hydrogen, is necessary. However fuel cell can provide only electrical energy. This electric energy has to be converted into mechanical energy by using the electric motor and inverter.
Benefits
- Low-to-Zero Emissions
- High Efficiency
- Reliability
- Fuel Flexibility
- Energy Security
- Ruggedness and Durability
- Scalability
- Quiet Operation
- Technology Compatibility
- Lightweight and Long-Lasting