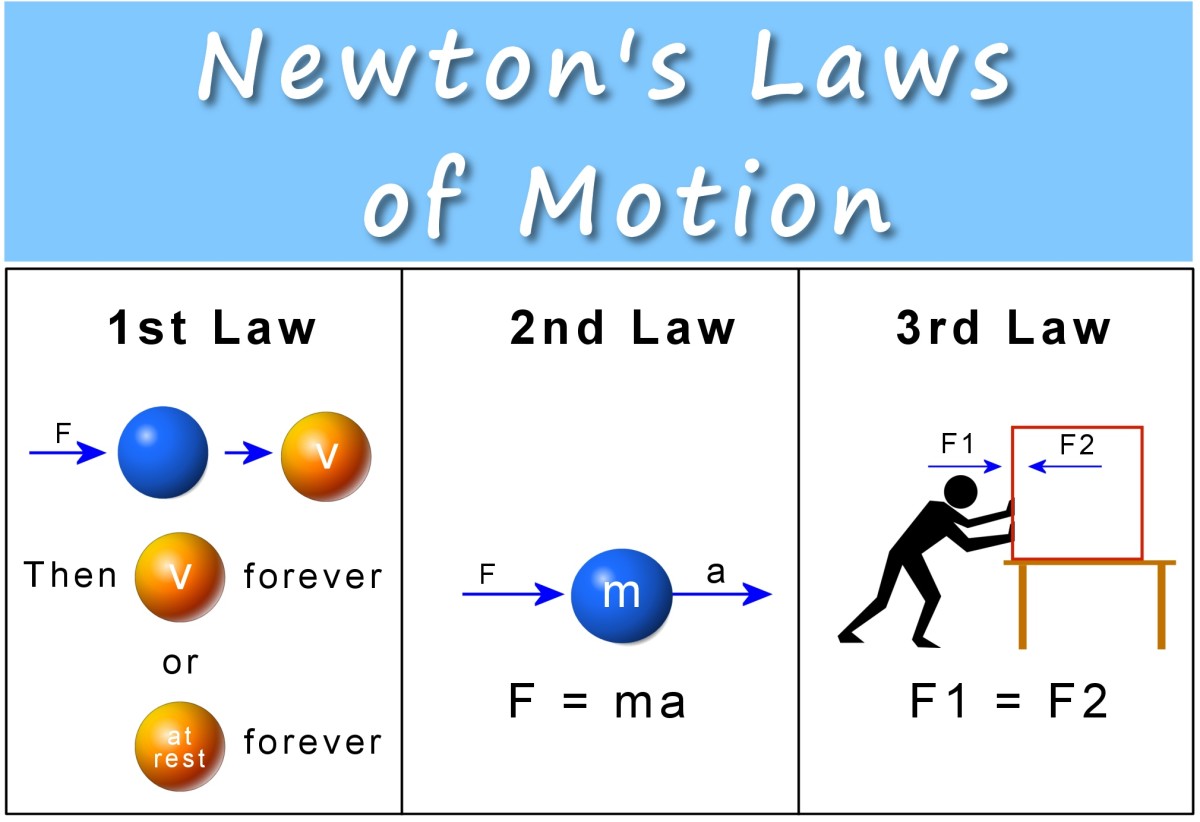
Some Words on Work and Energy


Energy
Yesterday, the kids were in the backyard in the dirt when I heard my daughter yell. I put down the dish I washed and ran out to see what was going on. She and her brother fought over some toys.
My reaction to my daughter yells began when I heard the yell and my nervous system told me to react. Then the potential energy stored within my leg muscles was put to use and I ran outside. Potential energy became kinetic energy as I began to move quickly into the backyard. This act of running outside was work, similar to the work of washing the dishes prior to my short jog.
There is a lot to be said about energy. For the purpose of this hub we will only look at how energy and work are related.
There is an obvious relationship since energy can be defined as the ability to do work. Kinetic energy is the energy of movement and potential energy is the energy an object has on hold until needed. We will stick with the definition given above that energy is the ability to do work.
To begin we must look at the
First Law of Thermodynamics:
Energy can never be created or destroyed but only transformed from one form to another. The total energy of an isolated system is always constant or the total energy of the Universe is constant.
The First Law of Thermodynamics has been an ongoing discussion throughout history. Thales of Miletus around 530 BCE mentioned conservation briefly and Empedocles (490-430 BCE) stated "nothing comes to be or perishes."
Galileo discusses conservation in his papers around 1638 yet conservation does not take the large step to becoming a law until Gottfried Wilheim Leibniz attempted to prove conservation through mathematics between 1676 and 1689.
Conservation had a roadblock that needed to be removed. Between the 1700's until the 1800's scientists and engineers tried to figure how to prove that energy was conserved and not lost. This finally was proven when the scientific community realized that energy was released in a system as heat and the amount of heat produced through work is hard to measure, especially if the heat is being released into the air.
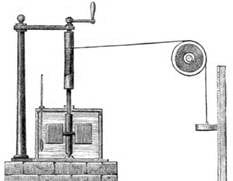
James Joule (1818-1889) was the first to measure heat released through work. He created an apparatus that had a falling weight pull a string to rotate a shaft in water. He then was able to calculate the change of temperature in the water after work was performed.
His calculation still stand today and 1 Calorie = 4.184 Joules.

Work
An object or system possessing energy has the capability to do work. When a system does work it loses energy and when work is done on a system energy is gained.
In a conservative system energy is transferred between kinetic and potential energy. Kinetic energy is the energy being used in the conservative system and potential energy is the work that can be used.
According to the Law of the Conservation of Mechanical Energy the sum of the kinetic energy and potential energy is constant when the system is conserved. Yet one must keep in mind that In a nonconserved system some energy is lost as a result of work being expended against frictional forces.
According to the Law of Conservation of Total Energy, even though energy is lost due to friction the total energy of the system is conserved.
The equation for work:
W = fd
W = Work
f = force
d = distance
or:
"Work done by something exerting a constant force is the product of the force along the displacment and magnitude along the displacement."
The SI unit of work is the Newton-meter (Nm) or the Joule (J).
In Conclusion
The example given in the beginning of this hub has me responding to my childrens yelling with work.
The word work has been given a bad rap. Most people when they think of the word, think of sweat and toil.
When in fact the use of energy in work is constantly occuring around us and through us. As work is being performed the energy used is released as heat that will usually dissipate into the air.
In open systems, like car engines, the heat created through work is removed through a cooling system. Some systems have been able to reuse the heat created as another source of energy.
As we think about the tasks and machines that we use everyday we should ponder about the energy being released as heat and think about a more efficient way to conserve.
Science has been looking at Conservation of Energy for hundreds of years and with our natural resources reaching points of depletion we should continue to look at work and energy.