AS Chemistry - Things You Need To Know - Part 2

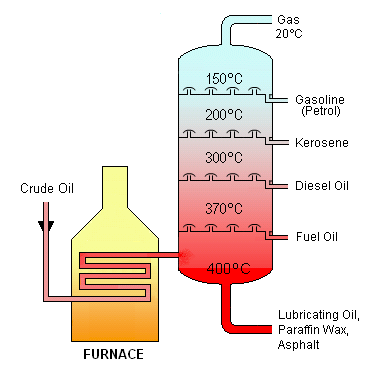
Crude Oil
- Crude oil is a fossil fuel that is been made from naturally decaying plants and animals that lived in the seas millions of years ago.
- In different places around the world the composition of crude oil differs - in some places there is lots of unbranched hydrocarbons and in other places there isn't.
- The average amount of different hydrocarbons in crude oil is 150 and most of these are unbranched.
- Crude oil as a whole does not ignite very easily, however, some of it's components are very useful and valuble and are used in various fuels and oils.
Fractional distillation:
How does it work?
1) The hydrocarbons in the crude oil are separated out into fractions.
2) Each fraction contains different hydrocarbons that have quite similar boiling points.
3) Further distillation of this fraction can obtain pure hydrocarbons.
Crude oil is refined in places called distillation plants but more specifically, fractional distillation takes place in something called a 'fractionating column'.
The fractionation column is hotter at the bottom than it is at the top so as the vaporised crude oil passes through it (and through a number of 'bubble caps') the gases reach a temperature that is cooler than that of their boiling point, thus they condense.
Long-chained hydrocarbons tend to have higher boiling points and so they condense near the bottom of the column (where it is hotter) and the opposite goes for short-chained hydrocarbons.
These fractions are then used in various things such as for fuel or petrochemicals.


What affects the boiling point of alkanes?
- As the length of the carbon chain increases so does the amount of interactions/contact between the different molecules and therefore the more van der Waal's forces there is between them. This means that it takes more energy to break the forces than a shorter chained alkane and the outcome of this is a higher boiling point.
- The more branched a molecule is then the lower it's boiling point will be. Molecules that are branched will not be able to get as close to neighboring molecules compared to unbranched alkanes and therefore the van der Waal's forces between the molecules will be weaker.
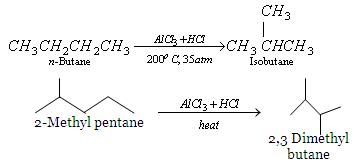

Uses of hydrocarbons - Fuels
Short-chained alkanes are valuable but are in very high demand. They can be used in fuels or polymer production. Referred to as 'clean fuels' they burn in plentiful amounts of oxygen to form CO2 and water. It is mainly used to fuel domestic heating and cooking - for example in barbecues and portable camping cookers.
Octane is a compound that is found in petrol and is used to fuel the internal combustion engine.
In reality, the car's internal combustion engine does not have enough of an oxygen supply for all of the octane to undergo complete combustion. This means that some incomplete combustion occurs and the resulting product is carbon monoxide instead of carbon dioxide.
Carbon monoxide prevents oxygen from binding to the haemoglobin and therefore is very dangerous and essentially fatal.
Branced and cyclic alkanes promote more efficient combustion compared to straight chained alkanes. The more efficiently a fuel burns, the higher the 'octane number' it will have (fuels close to 100 burn very efficiently).
Converting hydrocarbons - processes
- Cracking is a process that is used to break down long-chained hydrocarbons to form a mixture of shorter chained alkanes/alkenes. Most catalytic cracking processes use a zeolite catalyst and a temperature of about 450 degrees.
- There is a process that is sometimes referred to as isomerisation which is when straight-chained alkanes are converted into branched alkanes.
- A process called reforming can convert open (aliphatic) chained hydrocarbons into cyclic hydrocarbons.


The environment and fuels of the future
The earth's deposits of crude oil are dramatically depleting, and fast! This is due to the fact that over 90% of crude oil is used as fuel all over the world and the other 10% goes into making things like plastics and pharmaceuticals. As you can see, crude oil is pretty much essential to our everyday lives.
Burning hydrocarbons leads to an abundance of carbon monoxide, carbon dioxide, nitrogen oxides and sulfur dioxide polluting our atmosphere (read more about the chemistry behind global warming here).
A biofuel is a fuel that is derived from recently living material, instead of dead plants and animals from millions of years ago.
Some examples of things that can maybe be used as biofuels include:
- Agricultural crops such as sugar cane..
- Ethanol that is made from fermenting sugars/carbohydrates. It can be added to petroleum and make it burn more efficiently.
- Bioethanol.
- Biodiesel - an oil derived from plants the most common being rapeseed.