How Nuclear Fusion Keeps the Sun Shining
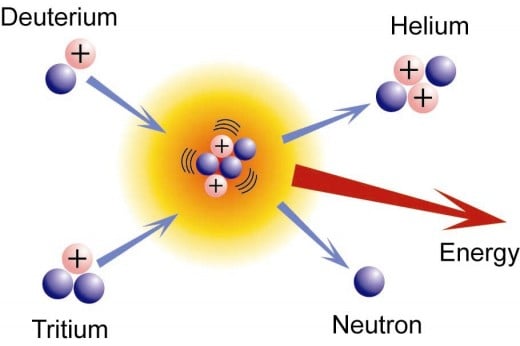
The foundation of solar energy is fusion of hydrogen to make heavier elements and isotopes
The sun generates electromagnetic radiation of all wavelengths by fusing light elements into heavier elements with the emission of energy as the result. The sun is in constant balance between gravity and electromagnetic pressure that keeps the whole process going as long as fusible elements are available to generate energy. The sun is 333,000 times the mass of the Earth and core temperatures are some 15,600,000 Kelvins. At the core, the density is some 13 times that of lead. These conditions are right for fusion of hydrogen.
At the core, whole atoms do not exist. The state of the solar interior is a dense plasma of free protons, neutrons and electrons. The energy of gravity created heat is intense, causing the occasional head on collision of protons. The usual process is one of the protons loses its charge to become a neutron. The emitted positron is absorbed by an electron creating gamma radiation in a matter-antimatter collision. This energy keeps the core hot and keeps the sun from collapsing due to gravity. The amount of energy created in just one of these events is measured as 27.76 Million electron volts. The product of fusion is a nucleus of deuterium, an isotope of hydrogen with one proton and one neutron. The next step up is the fusion of two deuterium nuclei to form an Alpha particle or Helium 4 nucleus according to the famous Einstein equation E = mc^2. The fusion produces energy and the nucleus loses about 0.7 percent of its mass. But it is not always so straight forward. Sometimes a tritium nucleus, a hydrogen nucleus with one proton and two neutrons, interacts with a single proton with the same result. This reaction is exceedingly rare. Alternately, a deuterium nucleus fuses with another proton to form a helium-isotope 3 nucleus. Every second the sun processes some 700 million tons of hydrogen into helium "ash". Some five million tons of mass "disappears" into energy which moves at the speed of light. But being trapped in the sun, the photons bounce around a lot and take a very long time getting to the surface. Every time a gamma ray encounters a nucleus or an atom, it losses some energy and its wavelength lowers until at the surface, it escapes as visible light. Sometimes X-rays and ultraviolet light escape in Coronal Mass Ejections. They help keep the reaction going and the sun in balance. When the energy finally reaches the surface, it escapes into space and the sun losses a little mass. Earth captures a tiny amount and life as we know it exists as a result.
Though the sun produces prodigious amounts of energy in the 4.56 billion years of its life, it still has only lost about .03 to .04 percent of its entire original mass. Modern observation has revealed that the sun is accumulating mass by sweeping up comets and interstellar dust, thus renewing itself somewhat. There is some concern about the sun exhausting its hydrogen fuel. Today about 37 percent of that hydrogen has been spent in the core. Since this type of hydrogen reaction will one day run out, the sun will exhaust itshydrogen fuel in about 7 billion years. The sun will then go through dramatic evolutionary change.
Today, the sun comprises about 92.1 percent hydrogen, 7.8 percent helium and .1 percent of all other elements according to the standard model. But there is no unanimous agreement as to the exact composition of the sun as we can see only the surface layers and calculate by analyzing Fraunhoffer absorbsion spectra. Some even suggest that the sun has a large iron core due to being a metal rich star. We have no way of really knowing. We do have a clue though in the form of neutrinos. A neutrino is an almost masseless ghostlike particle that can penetrate the solar mass and earth as if they don't exist. On rare occasions, they interact with atoms creating a reaction, emitting photons. According to predicted levels on the standard model, the sun is only producing 42 percent of the expected neutrinos. This is known as the solar-neutrino problem. It suggests that either our understanding of neutrinos or the standard solar model needs revision.
Some 2.5 to 3 billion years from now, the sun will go through a dramatic expansion once the hydrogen in the active core is used up. The sun will expand into a red giant, swallowing Mercury. Venus will barely escape. Venus, Earth and Mars will see their orbits expand and years lengthen as the sun losses mass by ejecting some of its outer layers. The sun will loom large in Earth's skies and no life will exist. Humanity may have reached the stars by this point, or have gone extinct with everything else that was once living. Once this point is reached, the sun contracts and the helium flash results where the sun now burns helium at its core. When the temperature in the sun’s core reaches 100 million Kelvins, helium fuses into carbon. Due to turbulence with outer layers, some left over hydrogen mixes and elements between helium and carbon in mass, like Lithium are formed. Oxygen if formed when helium fuses with carbon. Over the next three to four hundred million years, the sun will go through various expansions, contractions and flashes as heavier and heavier elements are made from the ashes of previous reactions. These elements being formed will be neon, sodium, magnesium, sulfur, silicon and nickel. The sun is not massive enough to form elements beyond this unless by chance, light elements fuse with already existing heavy elements like iron and gold. For a star more than three times as massive as the sun, the whole process ends when the iron stage is reached. Iron releases no energy either by fusion or fission and represents the bottom of the energy well. That star will end its life in a super-nova. The sun is not massive enough to fuse lighter ashes into iron. Thus it will never go nova. The sun will "quietly" shed mass and eventually become a white dwarf and then very slowly fade to black.
References:
The New Solar System 4th ed., Sky Publishing Corp. ISBN 0-933346-86-7, pages 25,37-38
The Scientific Companion by Cesare Emiliani, John Wiley and Sons, ISBN 0-471-62483-7, pages 50, 51