How Plants Deal with Stress and How Genetic Engineering Methods Might Enable Such Traits to be Transferred to Crops
Levitt (1972) defined stress in a biological context as being “any change in environment conditions that may reduce or adversely affect the normal growth and development of an organism”. This stress is able to exist because each organism has evolved to adapt to its particular environment and the range of conditions which exist there. When an organism is outside this optimum range of conditions, it is not adapted for this exposure and so encounters stress [Table 1]. Different organisms have different stressors (for example, fire will kill the majority of organisms however it is necessary for the seed germination of Pterocarpus angolensis) and even the same organisms can respond in different ways to the same stress (for example, due to the age or the stage of development of the particular organism) (Banda et al., 2006).
Plants, in particular, are able to adapt to stressors if they are exposed gradually, for example cold hardening (where plants are put outside for an hour per day and then this time is built up over weeks). They can cope with stressors either through avoidance or tolerance. Plants have sessile growth and so are less able to move away from the source of the stress, for avoidance, unlike animals who are able to adapt their behaviour to survive, e.g. by living nocturnally. Instead, plants can take some limited and slow evasive action by growing away from the stressor, for example, the roots of the plants in the Eastern Alps of Europe grow around or away from heavy metals in the soil (Punz and Sieghardt, 1993). Long term stress can also be avoided through specialised adaptive mechanisms, e.g. xerophytes avoid water loss by keeping their stomata closed. However, when the stress is too great to be avoided, it can be tolerated. A tolerant plant is able to withstand full exposure to an environmental stress with minimum adverse impact to the organism. It usually presents itself as a long-term adaption to a stable and frequently occurring set of environmental conditions (for example, the cold of winter), however stress can often be sporadic (for example, although the plants of Australia are adapted to forest fires, if humans prevent them, undergrowth expands causing a much larger fire which these plants cannot survive against).
Table 1 Abiotic Stressors, How Plants Cope with the Stress and How Genetic Engineering May Transfer These Traits to Crops
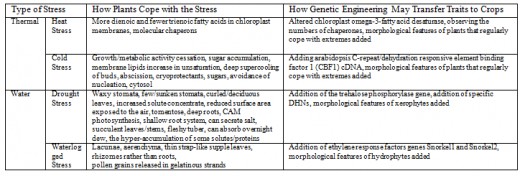
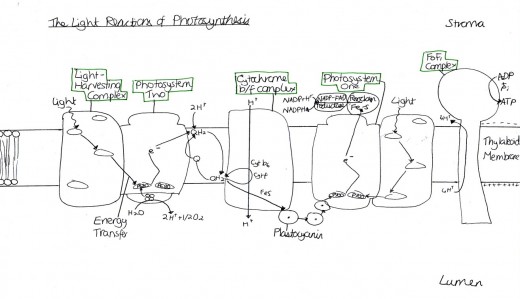
Figure 1 Light Reactions of Photosynthesis
Click thumbnail to view full-size
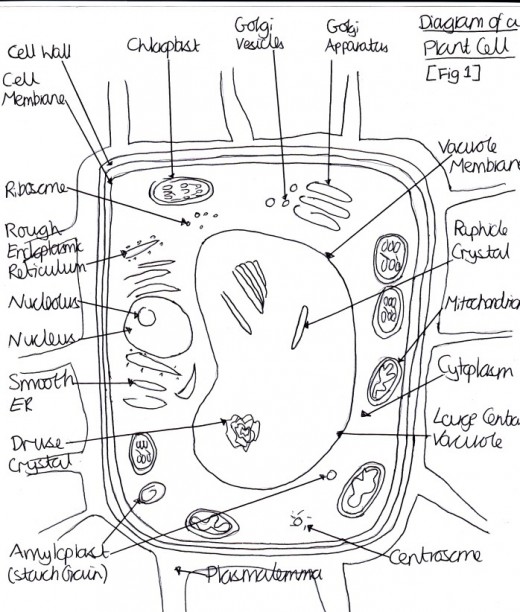
Figure 2 Diagram of a Plant Cell
Click thumbnail to view full-size
Thermal Stress
Thermal stress is reached when multicellular eukaryotes have a temperature of around 50oc or when plants reach the normal thermal death threshold of 45-50oc. The response to heat stress is dependent on: the duration of exposure, the age of the plant’s tissue, water content of the plant and environment and the adaptability of the plant.
Heat Stress
Heat stress syndrome is a series of metabolic dysfunctions and physical constraints that accumulate until the plant can no longer adjust, avoid or correct, to protect itself against the stressor (Batanouny, 1936). The process in C3 plants, such as trees, occurs over several days or weeks and begins with a decrease in photosynthesis and an increase in respiration, so the plant is using energy but not producing any [Figure 1]. To increase photo-respiration and stop carbon dioxide capture, the stomata close, which reduces the rate of transpiration, making the internal temperature increase because of the termination of the cooling process. This then causes cell membrane leakage because the plant membrane becomes more fluid, causing decreased permeability and ion leakage, which causes signal changes in protein synthesis. This occurs because the lipid components are usually semi-fluid and so are susceptible to increasing fluidity, which causes them to separate. After this, continued physical water loss occurs because of this membrane leakage. In an attempt to conserve energy, the growth processes are inhibited. Next, starvation occurs because of inefficient food use and an inability to call on reserves (such as starch) when they are needed, since the phloem are impeded. After, the toxins that were previously confined to vacuoles are released through the cell membrane and respiration problems occur [Figure 2]. Then, the membrane integrity is lost and proteins are irreversible denatured because the protein sub-units are dissociated and the spatial arrangement of protein subunits is disrupted, since the covalent interactions between amino groups, noncovalent dipole-dipole interactions and Van der Waals forces are interrupted. The proteins that denature include key enzymes and pigment/protein complexes involved in photosynthesis (for example, Photosystem II in the thylakoid membranes of the chloroplasts is highly sensitive to increases in temperature [Figure 1]).
The sensitivity of photosystem II to heat may be due to the detrimental effects of increased temperature on the membrane of the chloroplast [Figure 1; Figure 2]. Plants are able to naturally maintain life by increasing the saturated fat proportions in their membranes. This is because saturated fats are more rigid, so additional heat is required to cause membrane fluidity. Genetic engineering strives to produce the same, but enhanced, effects. Murakami et al. (2000) developed transgenic tobacco plants which altered chloroplast omega-3-fatty acid desaturase. This caused plants to have more dienoic (two double bonds) and fewer trienoic (three double bonds) fatty acids in their chloroplast membranes, which allow the fatty acids to stack more closely to decrease the fluidity of the membrane since they are more saturated. However, these plants were only tested in artificial environments and so further testing in natural environments is required. Furthermore, the genetically modified plants, as a whole, must be taken into consideration because there may be wider consequences of the removal of the trienoic fatty acids, for example, possible reproduction repercussions.
The plant can also increase the number of structural proteins, which hold the membranes together, synthesised. These molecular ‘chaparones’ physically protect proteins (e.g. photosystem II) from stress by binding to them, or target proteins for degradation when they have been damaged. Lee and Vierling (2000) also found that the chaperons are able to repair proteins and ‘reactivate’ heat-damaged proteins. However they cost energy to be produced and so are only made if necessary. Thus, cowpeas were bred to have different levels of heat tolerance to observe the effect of the heat-shock proteins. The results found were that all the cowpeas had the same set of heat-shock proteins, therefore heat tolerance is this way must be due to multiple genes within the genome and genetic engineering is a more complex process than what was initially thought.
Cold Stress
Chilling injury usually occurs when a plant has a temperature of 20-0oc and can cause physiological disruptions in: germination, flower development, fruit development, yield, and storage life. Kratsch and Wise (2000) found the ultastructural systems of chilling injury: the chloroplasts and mitochondria swell and are disorganised, smaller and fewer starch granules, thylakoids dilated, grana unstacked, small vesicles of chloroplast peripheral reticulum are formed, lipid droplets are accumulated in chloroplasts and the chromatin in the nucleus is condensed. Cold acclimation consists of: growth cessation (because of the ABA accumulation in buds and leaves), mutable activity cessation, sugar accumulation (including sucrose and other polysaccharides), membrane changes (for example, the lipids within it may increase in unsaturation) and sometimes deep supercooling of buds and (if deciduous) abscission. It begins when environmental cues are recognised, such as shortening day length and decreasing temperatures.
Freezing injury happens when the external temperature is below 0 oc, thus both the intracellular and extracellular parts of the plant are at risk of freezing. However water is able to be a liquid at -47 ºC without nucleating and forming ice, but when nucleation of the supercooled water does occur, intracellular ice forms suddenly resulting in plant death.
Intracellular freezing, or rapid freezing, damages the protoplasmic structure and the ice crystals kill the cell because water cannot exit the cell fast enough to equilibrate. It can be slowed by cryoprotectants e.g. DMSO and glycerol. Extracellular freezing, or slow freezing, dehydrates the protoplasm of the plant because cellular water forms ice crystals, causing a water-vapour deficit so the water moves down the concentration gradient, which is known as freeze dehydration. It can be slowed with sugars. Sugars have a high concentration of protective solutes and so shrink less to reach equilibrium, losing less water. This means that the membrane is stabilised and the relative concentrations of salts and damaging metabolites is decreased.
Plants are adapted to be able to cope with freezing because they can stop the formation of intracellular ice crystals. Hsieh et al. (2004) investigated this by genetically modified tomato plants by adding arabidopsis C-repeat/dehydration responsive element binding factor 1 (CBF1) cDNA driven by a cauliflower mosaic virus 35S promoter. It was found that the transgenic plants exhibited a greater degree of chilling tolerance compared with wild-type plants. Thus, research into this area is still on-going and looks promising. Plants also cope with cold stress by: cessation of metabolic activity, the avoidance of nucleation sites so that ice cannot form, they have cryoprotectants (for example some sugars and polyhydric alcohols) and cytosol to lose much of their water while remaining intact. Possible genetic engineering could allow plants not adapted to the cold to have these features in future.
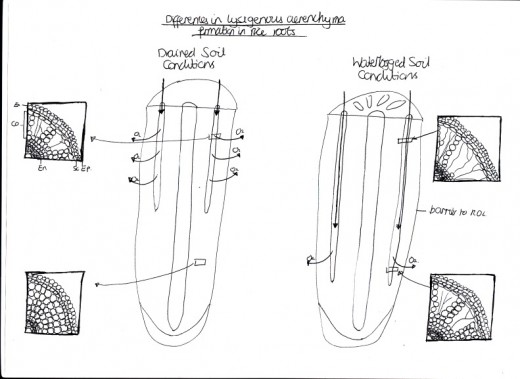
Figure 3 Differences in lysigenous aerenchyma formation and patterns of radial O2 loss (ROL) in rice roots under drained soil conditions and waterlogged soil
Click thumbnail to view full-size
Water Stress
Having too much or too little water is a common occurrence in plants, meaning that they are well adapted to a wide range of water stresses, for example summer droughts occur in the UK but the plants can survive for a few weeks without water, and the same for floods. Regardless, there are plants that are adapted to extremes of water stresses.
Drought Stress
Xerophytes are plants that grow in environments where water is scare, whether that is dry deserts or Mediterranean-type climates with intermittent rainfall or in Arctic conditions, where the water is frozen. It is important to conserve water in these environments because if the water vapour potential inside the leaves of a plant is greater than outside, it will diffuse out through the stomata and down the concentration gradient, which is known as transpiration. This loss of water can cause a plant to lose turgor and plasmolysis will occur (when the protoplast in the cell wall contracts, moving away from the cell wall and causing gaps to form between the cell wall and membrane). Eventually, cytorrhysis occurs, which is the irreversible collapse of the cell wall, resulting in cell death, so the plant itself would wilt and die. The adaptions of xerophytes are to prevent this process. One such adaption is the reduction of surface area exposed to the air, to reduce the amount of evaporation e.g. the pines of cacti are reduced leaves. Another adaption is that of tomentose, which is to have small hairs on the external surfaces to provide a wind break and reduce air flow to reduce the rate of evaporation. Water loss is also minimised using the following adaptions: waxy stomata, few stomata, sunken stomata, curled leaves, deciduous leaves (do not require energy throughout the year because can be dropped) and an increased solute concentrate (to alter the concentration gradients). Water uptake is maximised by having deep roots that can penetrate over 20m to the water table. The amount of water stored is increased by succulent plants that have Crassulacean acid metabolism photosynthesis (so stomata can be closed during the day because the plant is able to produce its own carbon dioxide through the decarboxylation of malic acid), a shallow root system, the ability to secrete salt as a method of excreting waste metabolites, succulent leaves and stems, a fleshy tuber and the ability to absorb overnight dew e.g. from fog. To conclude, genetic engineering could possibly alter existing plants to have these morphological characteristics, however dehydration tolerance is still not greatly improved. For example, an experimental strain of genetically engineered corn has been tried in Kansas. It is known as DroughtGard corn and carries a gene to help the plant draw water more gradually from the soil (Plumer, 2012).
Although the mechanism of dehydration tolerance is poorly understood, the hyper-accumulation of some solutes (e.g. trehalose) and proteins (e.g. dehydrin) have been researched in euxerophytes. Han et al. (2005) found that tobacco plants that were genetically engineered to produce and contain trehalose were more resistant to drought, e.g. after 10 days without watering, the plants were still growing and functioning normally. The trehalose phosphorylase gene was taken from Pleurotus sajor-caju. Thus, this process is being tested in different crops to observe if similar results are found. Layton et al. (2009) focused on a different mechanism and used Western Blotting to find that dehydrins were present in those plants most able to withstand dehydration and were most prevalent in the cell walls. These proteins have a negative charge and so are able to attract, sequester and localize water. There is a future possibility that crops could be genetically engineered to synthesise these proteins themselves. Ruibal et al. (2012, pp1), agrees with this theory, saying “results suggest that specific DHNs contribute considerably to the high stress tolerance of mosses and offer novel tools for genetic engineering stress tolerance of higher plants”.
Waterlogged Stress
When plants are saturated with water, they usually wilt and then die because the soil become anaerobic, making the roots rot. Hydrophytes are plants grow constantly in water and so have become adapted to a waterlogged environment. Air-filled spaces are found in hydrophytes, known as lacunae in leaves and aerenchyma in other tissues, allowing organs to keep floating and the plant to obtain sufficient light for photosynthesis [Figure 3]. This is because upright leaves get more light than sunken leaves (aid in flotation). Another adaption is that up to 60% of the volume of the leaf is air space to allow for gas exchange throughout the plant. By having thin, strap-like, supple leaves, it allows the hydrophytes to withstand tidal currents and the wave action of the waters within their environments because they can move with the flow of the water. In addition, some species have rhizomes rather than roots for attachment to the soil (or bed), to allow fast vegetative growth. Reproduction is also altered because pollen grains are released in gelatinous strands that are carried by the currents of the water.
The use of genetic engineering has been extensively studied in rice plants because of the concern of flooding in rice paddies. There are two main types, depending on the type of water stress experienced: quiescence and elongation (Luo et al. 2011). Sarkar and Bhattacharjee (2011) said “the ethylene response factors genes Snorkel1 (SK1) and Snorkel2 (SK2) allow rice to adapt to deep water whereas Submergence1A-1 (Sub1A-1) allows rice to acclimatize under flash flooding”. Both types are connected with gibberellin biosynthesis or signal transduction, but can also avoid the water by elongation or remain stunted under water until flood recedes. However, this is only for short term exposure to large amount of water, thus further research is necessary.
References
Batanouny, K. (1936), Plants in the Deserts of the Middle East, Chapters 6-7, Springer, Egypt.
Banda, T., Schwartz, M and Caro, T. (2006), ‘Effects of Fire on Germination of Pterocarpus angolensis’ Forest Ecology and Management, Volume 233, pages 116–120, Available at http://www.des.ucdavis.edu/faculty/mschwartz/Website%20publications/Banda_Seed_Germ.pdf, (Accessed 12/02/13).
Han, S., Park, S., Kwon, H., Yi, B., Lee, G. and Byun, M. (2005), ‘Genetic Engineering of Drought-Resistant Tobacco Plants by Introducing the Trehalose Phosphorylase (TP) Gene from Pleurotus sajor-caju’, Plant Cell, Tissue and Organ Culture, Volume 82 (issue 2), pages 151-158, Available at http://link.springer.com/article/10.1007%2Fs11240-004-8124-1?LI=true, (Accessed 22/02/13).
Hsieh, T., Lee, J., Chiu, L., Charng, Y., Wang, Y. and Chan, M. (2004), ‘Heterology Expression of the Arabidopsis C-Repeat/Dehydration Response Element Binding Factor 1 Gene Confers Elevated Tolerance to Chilling and Oxidative Stresses in Transgenic Tomato’, Plant Physiology, Volume 129 (issue 3), pages 1086-1094, Available at http://www.ncbi.nlm.nih.gov/pubmed/12114563 , (Accessed 22/02/13).
Layton, B., Boyd, M., Tripepi, M., Bitoni, B., Dollahon, M. and Balsamo, R. (2009), ‘Dehydration-Induced Expression of a 31-kDa Dehydrin in Polypodium polypodioides (Polypodiaceae) May Enable Large, Reversible Deformation of Cell Walls’, American Journal of Botany, Volume 97 (issue 4), pages 535-544, Available at http://www.amjbot.org/content/97/4/535.full, (Accessed 22/02/13).
Levitt, J. (1972), ‘Responses of Plants to Environmental Stresses’, Academic Press, New York. Punz, W. and Sieghardt, H. (1993), ‘The Response of Roots of Herbaceous Plant Species to Heavy Metals’, Environmental and Experimental Botany, Volume 33, (Issue 1), pages 85–98, Available at http://www.sciencedirect.com/science/article/pii/009884729390058N, (Accessed 12/02/13).
Luo, F., Nagel, K., Scharr, H., Zeng, B., Schurr, U. and Matsubara, S. (2011) ‘Recovery
Dynamics of Growth, Photosynthesis and Carbohydrate Accumulation After De-submergence: A Comparison Between Two Wetland Plants Showing Escape and Quiescence Strategies’, Annual of Botany, Volume 107, pages 49–63.
Murakami,Y., Tsuyama,M., Kobayashi, Y., Kodama, H. and Iba, K. (2000), ‘Trienoic Fatty Acids and Plant Tolerance of High Temperature’, Science, Vol. 287 no. 5452 pp. 476-479, Available at http://www.sciencemag.org/content/287/5452/476, (Accessed 12/02/13).
Plumer, B. (2012), Farmers turn to engineered corn to adapt to drought. But will it be enough?, The Washington Post, Available at http://www.washingtonpost.com/blogs/wonkblog/wp/2012/08/15/farmers-turn-to-engineered-corn-to-adapt-to-drought-but-will-it-be-enough/, (Accessed 22/02/13).
Kratsch, H. and Wise, R. (2000), ‘The Ultrastructure of Chilling Stress’, Plant, Cell and Environment, Volume 23 (issue 4), pages 337-350.
Ruibal, C., Salamo, I., Carbello, V., Castro, A., Bentacor, M., Borsani, O., Szabados, L. and Vidal, S. (2012), ‘Differential Contribution of Individual Dehydrin Genes from Physcomitrella patens to Salt and Osmotic Stress Tolerance’, Plant Science, Volume 190, pages 89-102.
Sarkar, R. and Bhattacharjee, B. (2011), ‘Rice Genotypes with SUB1 QTL Differ in
Submergence Tolerance, Elongation Ability during Submergence and Re-generation Growth at Re-emergence’, The Rice Journal, Volume 5 (issue 7), Available at http://www.thericejournal.com/content/5/1/7, (Assessed 22/02/13).