Myriad BRCA1/2 patents: A thin line between commerce & consumer health
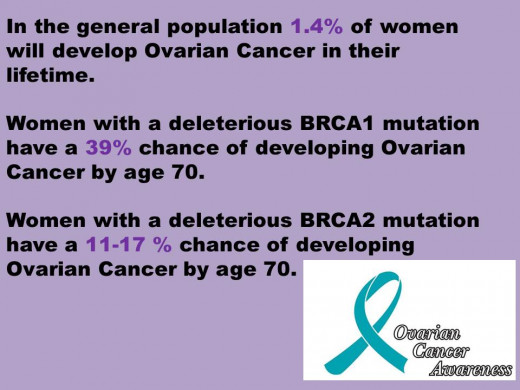
Women who have a first degree relative with Breast or Ovarian cancer may have an increased risk of developing hereditary cancer and thus BRCA1/2 analysis is recommended for them.
Over the summer, a rather hot topic has been discussion surrounding the Supreme Court case deciding the fate of the biotechnology company Myriad’s patents of the genetic information contained with the BRCA1 & 2 genes. As with any debate there are always two sides to every story; the additional layer here is the two-headed biotechnology beast- combating disease and making money. Here are the facts- in the early 90s researchers from the University of Utah set up the molecular diagnostics company, Myriad Genetics Inc. Work done around the same time, by both researchers in this group and others as well (see timeline), allowed for the discovery of the BRCA1 and 2 genes, along with their isolation and sequencing. These two genes, whose abbreviations stand for Breast Cancer, early onset 1 & 2 have been found to increase a women’s chance of hereditary breast and/or ovarian cancer.
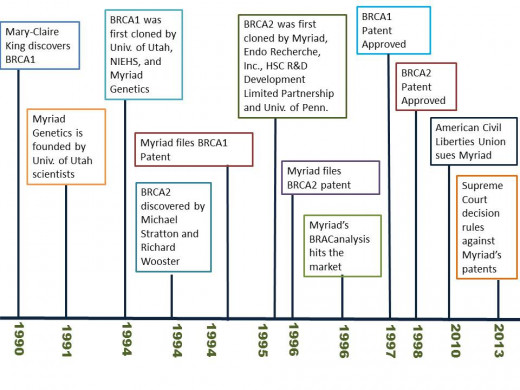

Myriad went on to develop a genetic test panel to explore an at-risk woman’s chances of breast or ovarian cancer by analyzing the individual woman’s BRCA genes for mutations. Here’s where we get to the controversial part- in order to recoup the money and time spent on discovering these genes and developing their test panel- Myriad filed patents for both the isolated BRCA genes and strong-armed any institution, academic or otherwise, that tried to run diagnostics on women’s BRCA genes as well to assess their cancer risk in a commercial venture, allowing Myriad the sole legal right to test these two genes in the US.
Myriad patented the isolated DNA that codes for the BRCA1 and BRCA2 genes, meaning that anyone outside of Myriad walls that wanted to test BRCA1 and 2 by growing up the gene products, the proteins the DNA coded for, they would be doing so illegally. While these patents were being enforced, Myriad did partner with many academic sites to engage in collaborative BRCA research but it seemed that if Myriad didn’t want you doing the research then you weren’t. The company insists it had no intention of preventing academic institutions from researching the breast cancer genes. Unfortunately a well publicized battle took place between Myriad and an academic lab, University of Pennsylvania Genetic Diagnostics Lab, since Myriad claimed the lab was trying to sell a diagnostic test for BRCA1/2. This led many other researchers to believe it was best to stay away from breast cancer molecular research, which is a shame. In addition public image research was conducted in 2007 showing that 78% of article about Myriad and its BRCA patents were negative, 16% were neutral and only 6% of the reporting showed Myriad in a positive way (2).
Do you think Myriad has a right to enforce its exclusivity on BRCA1/2 testing?
I worked at a biotechnology company for five years and in that time I formulated my thoughts on this industry. There is a fine balance between serving the customer and being a profitable business. Ultimately a company should have its initial focus on the former, but if they don’t also have a focus on the latter than they would not be able to help the customer going forward. The bottom line is companies need to stay in business. To some extent that is what the president of Myriad said in a press release that was posted on the company’s website in April 2013 in response to the Supreme Court case. This company invested so much time, effort, and money into discovering the genetic clues behind breast and ovarian cancer. Because of this company there is a diagnostic test that allows women to gauge their risk of hereditary based breast and ovarian cancer. It is only right to allow Myriad the time to market this test without introducing competition so that they can make back some of the money they initially put into this project. This ensures the test will continue to be made available to the public and also it promises that this test will not be the last contribution made by this company. (Myriad also has genetic tests for prostate, colon, skin, and pancreatic cancer). I think the question isn’t about whether Myriad had a right to patent the BRCA sequences, at the time this was an acceptable method of discovery protection. I think the question is did they go too far enforcing their rights?
In 2009, a suit was filed against Myriad by the ACLU (American Civil Liberties Union) and the Public Patent Foundation, on behalf of the Association of Molecular Pathology. The case was ultimately appealed to highest system and the Supreme Court issued their ruling on June 13th 2013. The decision revoked Myriad's patents on BRCA1&2 as well as set the new precedence of what researchers can and cannot patent in the future. The ruling prevents any future patents being issued on genes that are isolated from the human body but otherwise unchanged by the researchers. We'll get back to this last point in a moment but first how the decision affects Myriad.
One way or another Myriad’s BRCA patent’s days were numbered, whether the Supreme Court ruled against them or not. Patents expire after twenty years, the BRCA1/2 patents were set to expire around 2015. Myriad has gone on to patent other portions of the overall genetic testing procedure so that their exclusivity should hold regardless of these specific patents. Myriad has another ace up its sleeve to help maintain market dominance, VUSs- Variants of Unknown Significance. Here’s the deal with BRCA mutations- there are hundreds of them that lead to cancer risk which makes the task of sorting and classifying BRCA data all the more complicated.
There are three populations of BRCA mutations-
- clearly deleterious (causes cancer)
- clearly neutral
- VUSs (Variants of Unknown Significance)-a catch-all for those sequences that can’t yet be put into the other two categories.
Myriad initially opted to share its VUS data, based on its patient’s test results, in public databases but then abruptly stopped sharing it. Keeping their proprietary VUS data under lock and key is suspected to be directly related to their BRCA patents coming to an end one way or another. Myriad credits the VUSs as the reason their testing is 97% certain, while other tests will return results that are about 60 to 80% accurate. This marks another strategic move that is met with mixed reactions of ‘That’s a wise business decision’ and ‘That completely goes against the collective discovery effort associated with disease research.’ I personally understand their decision but I think it’s unfortunate for cancer research. Part of me feels like they have already had their time to make their money back on the BRCA tests while the patents were recognized for the past 15 years. It would be a show of good faith to the research community to continue to share their VUS data. It seems like a schoolyard tactic to me, their previous patents made it so not many other institutions could collect valuable VUS data on the BRCA1 and 2 genes so by default they are the ones with the most data on these sequences and now that they don’t have full reign of the sandbox they are cowering in the corner not sharing any of their toys.
It should be noted that it is unlikely VUSs would lead to the identification of a bad news breast cancer mutation within the BRCA genes. Also in fairness to presenting both sides- Myriad insisted, at the time they stopped sharing data, it was because they didn’t want physicians to make the wrong assumption about these VUSs.
One opinion on what the Supreme Court decision means for future molecular testing
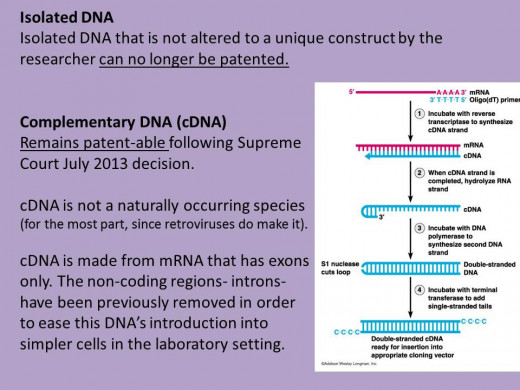
Regardless of where you stand on this issue the fact of the matter is the Supreme Court has now ruled on this and there are ramifications. The Supreme Court ruling stuck to the basics to make its decision- although Myriad put the time and effort into the discovery of the BRCA1&2 genes they did not invent them and thus cannot have a patent on them. This means going forward patents on human genes will not be approved. Prior to the ruling there were approximately 2000 other gene patents similar to the BRCA1/2 patents. These patents, if based strictly on isolated DNA, may be deemed invalid as well. It remains to be seen how this ruling will affect plant and microbial isolated DNA used for therapeutic purposes, since the Supreme Court opinion did not single out species one way or the other. The decision should free up genes for competitive clinical testing, since for the most part molecular diagnostic tests use isolated genes. Now that these isolated genes cannot be patented, a company cannot have a monopoly based on patents alone. However there is a wrench in all of this and the wrench is cDNA. The distinction between cDNA and isolated DNA was made in the ruling and cDNA is still eligible for patents. Since cDNA is also used in molecular diagnostic tests as well as isolated DNA future patents could be written to exploit this, likewise it’s possible many of the now-in-question patents will not be overturned if they are based on cDNA. The cDNA issue is important to Myriad as well, since the company still believes it has the exclusive right to test BRCA1/2 due to its other patents that were not voided. In fact, Myriad immediately filed suit against Ambry Genetics who wasted no time in advertising their own BRCA1/2 testing the day the Supreme Court ruled against Myriad. It remains to be seen if the ruling will end Myriad's dominance in BRCA testing or if the cDNA loophole will allow them for a continued monopoly.
Sources
1. Levy S. Our Shared Code: The Myriad Decision and the Future of Genetic Research. Environmental Health Perspectives. August 2013;121(8):A250-3.
2. So D, Joly Y. Commercial Opportunities and Ethical Pitfalls in Personalized Medicine: A Myriad of Reasons to Revisit the Myriad Genetics Saga. Curr Pharmacogenomics Person Med. 2013 Jun;11(2):98-109.
Additional resources available upon request.








