Why Does The Water Molecule Exhibit Polarization
Water Molecules Give Off Polarization
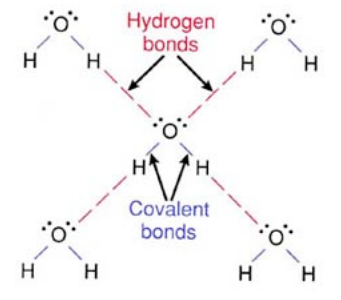
A water molecule contains 2 Hydrogen atoms and 1 Oxygen atom, which makes up the molecular formula os H2O.
It is because of these atoms and how they interact which gives water a polar characteristic which answers why does a water molecule exhibit polarization.
Covalent Bonds
Covalent bonds are extremely strong and are not broken easily unless under extreme energy such a stronger oxidizing agent or in the presence of a catalyst which lowers the activation energy required.
These covalent bonds exist within a water molecule.
Van Der Waals Forces
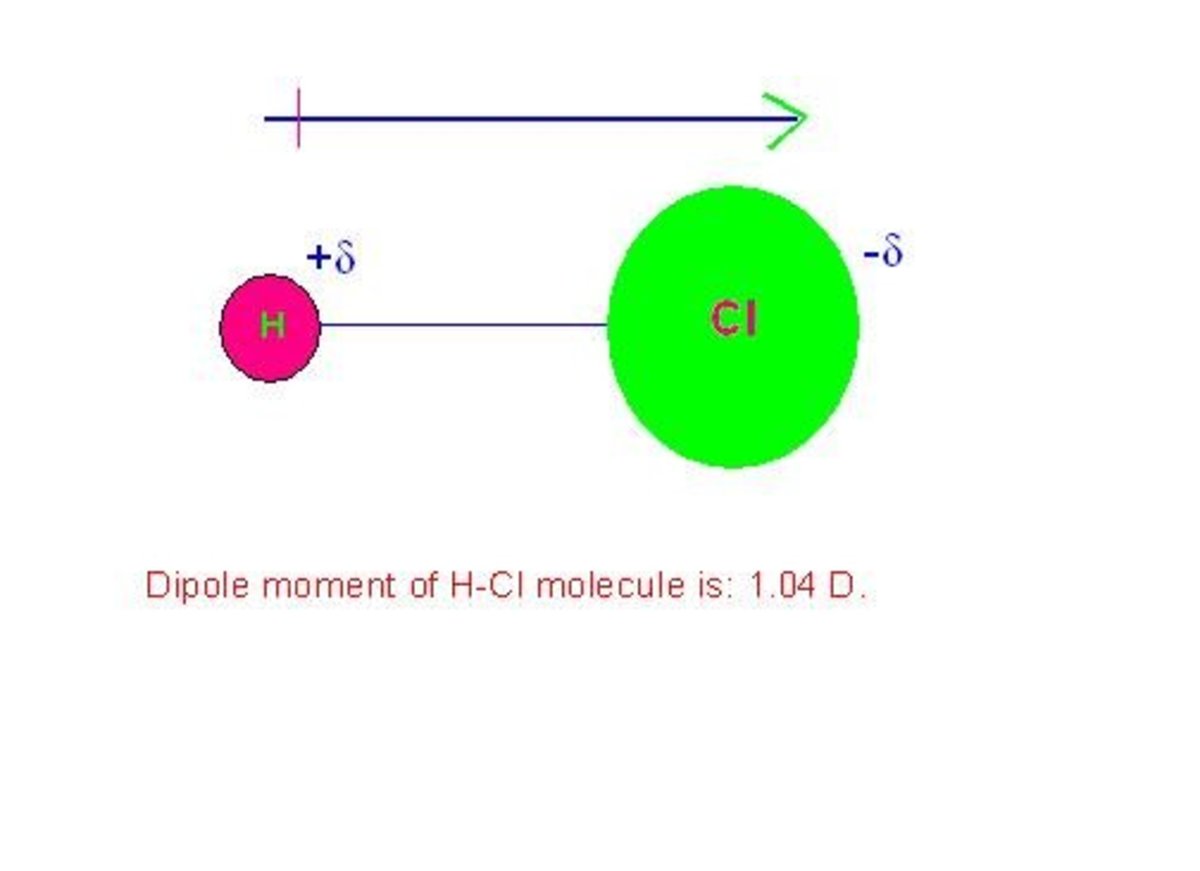
The electrons, which are the negative charges, are drawn towards the oxygen atom. This makes the oxygen atom slightly more negative, and the hydrgon atoms slightly more positive. As a result, a very weak “magnet” is formed, and the weak attractive force is given the name a “Van Der Waals” force. This is shown in the graph provided.
It is this electrotivity and the result of how electrons reside in a water molecule, by residing within the oxygen's electron orbital more, which gives weak inter-molecule forces making it a polar molecule. As a result, this is why the water molecule exhibits polarization.
More About Water
- How To Increase Testosterone Through Food
It is easy to increase testosterone through the food we eat. Perceived as the vital hormone for all round health, here are the best foods to increase testosterone naturally. - Is The Moon A Planet Or A Satellite
The moon is a satellite. The moon is neither a planter or a star. The term satellite is given to large masses that orbit a planet. As a result, the moon is Earth's naturally occurring satellite. - Why Does Water Pressure Increase With Depth
It is easily accepted that that water pressure increases with depth, and yet if we were to ask someone why does water pressure increase with depth, not many would be able to answer that question. Fortunately, after reading this article, you will be a - Why Does Water Expand When It Freezes