Fast and Easy Science Fair Projects: Water Maker







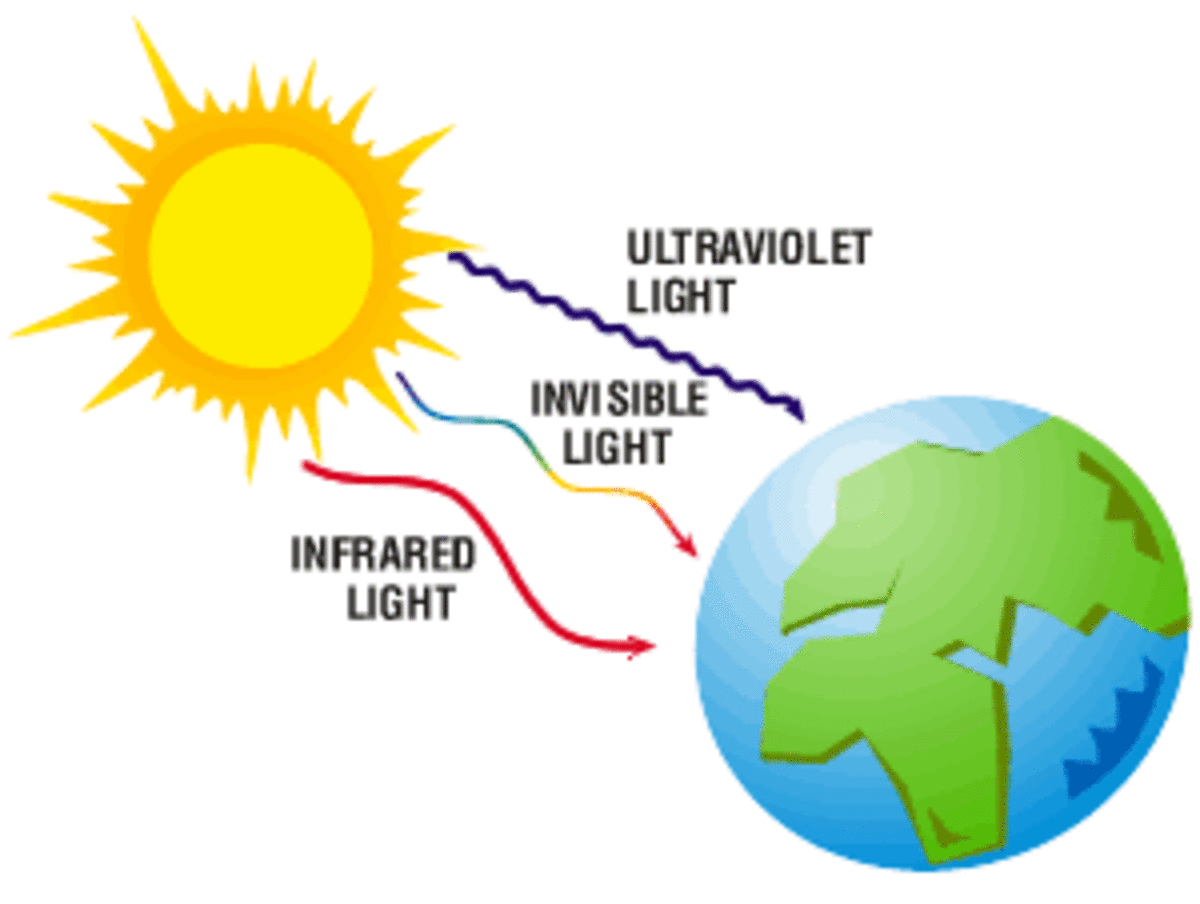
Solar Radiation
Purpose: How could we change snow to water if the temperature was below freezing?
Overview: If you were stranded in the woods or in a place where there was lots of snow and ice and you needed water to drink, how could you get it to melt?
On a sunny day when there is snow on the ground, you may notice the snow in places starting to melt, even though the air temperature is below freezing -3.2 degrees Farenheit or 0 degrees Centigrade. Where do you see this happening? Is the ice melting because the sun is hitting it? Would the melting stop if a black plastic bad was placed over it to block ths sunlight getting to it, or do you think the black color would collect even more sunlight and turn it into heat that would melt the ice faster?
Hypothesis: Hypothesize that a black covering will absorb sunlight and the hea will make the ive melt even faster.
You need:
- 3 clear cereal bowls
- 12 ice cubes
- clear plastic food wrap
- black plastic mash bag
- sunny day when the outdoor temperature are cold
- clock or watch
- paper and pencil
Procedure: Place three cereal bowls outside on a sunny, but cold, day. Pour four ice cubes in each bowl. Cover one bowl with clear plastic food wrap. Cover another bowl with a black plastic trash bag. Leave the third bowl uncovered. Our Variable is the color of the plastic covering. Temperature, location, amount of sunlight, and ice are held Constant. We are assuming that either the thickness of the food wrap and the plastic bag is the same or, if not the same, that the thickness will not affect the results. What do you think will happen to each bowl of ice?
After setting the bowls outside in a sunny place, check them every fifteen minutes. Write down your observations (recording what you see happening) in each bowl. if you don't see any change, write down that there is no change.
After a time, water may appear in some of the bowls. If ice appears in more than one bowl, you can find out in which bowl the ice melted faster by "quantifying" (measuring) the amount of water in each bowl by comparing the water in each bowl you remove the ice cubes.
Results and Conclusion: Write down the results of your experiment. Come to a conclusion as to whether ot not your hypothesis was correct.
Something more: 1. You may wish to continue your experiment by making covers of different colors, red, green, yellow, and others. Is there a difference in the amount of ice melted between a bowl of ice that is air tight (covered by plastic wrap) and a bowl that is air tight (covered by a piece of colored construction paper), even if both colors are the same?
2. Does the thickness of the cover act as an insulation? Try comparing various cover thicknesses.
Related Pages:
- Down-Range Shooter
Purpose: The purpose of this experiment is to determine the angle of trajectory that will give the greatest distance. Overview: When you throw a stone a little upward and away from you, you know that it... - Room for Brightness
Reflected Light Purpose: Show that a room is better lit when the room's walls are painted in bright colors compared to a room where the walls are dark (makes a room safer, (reduces eye fatigue when... - We are #2
Second- and third-class levers Purpose: Learn to use a second class lever (a simple machine) to reduce the force required to lift a heavy object. Overview: A lever is mage up of a long shaft or board... - An Uphill Battle
Kinetic energy and the transfer of energy Purpose: Demonstrate that energy can be transferred from object to object, and defy gravity. Overview: An energy force can travel like a wave, which means that it... - Bottled Force
Kinetic and Potential energy Purpose: Let's go if we can find a way to store "work" energy. Overview: Energy can be placed in one of two groups, kinetic energy and potential energy. Kinetic energy is...