Without sugar and oil, how much should I eat for preserving my weight?

Introduction
Astonishingly, a diet without bad fats and refined sugar allows us to increase the amount of foods (mainly in the raw forms) we can eat preserving (or loosing) our weight. Have you ever tried to eliminate vegetable oils (or salad dressings reach in fats and calories) and refined sugar from your diet? For instance, you can use pods anti-adherence instead of cooking oil and artificial sweeteners instead of refined sugar. In this possible reality, the only factor that would prevent you to be engorged by foods is the Fullness Factor associated with each food.
You must always be aware of few basics in the art of fueling your body: one teaspoon (1 tsp = 7 g) of olive oil is equivalent to about 45 cal (1 tbsp = 135 cal), one tsp of proteins is equivalent to about 16 cal (1 tbsp = 48 cal) and one tsp of sugar to about 16 cal (1 tbsp = 48). With this information in mind, you must think about what you are doing when you are dressing your salad with a little more olive oil (feeling the lightness of the stylized vegetables in your mind!) or when you are saturating your fruit juice (already reach in natural sugar) with more sugar. Obviously, you are risking to over mounting a light dish of calories.
Let us think about this risk in other terms. If I had avoided to use one tablespoon of oil in my salad, I could have had more salad or more dessert at the end of the meal (or whatever you like, but not oil!); or I could have had lost definitively those calories to reach my weight goal in a shorter time. Just one tbsp of oil! Not only for your weight, but for your vessels also!
Now let us think about this in another way. What if we eliminated completely oils, butter and sugar from our diet? You won’t believe how much freedom to eat you would gain eliminating few components of your diet!
And if we wished to shine in our diet we could select foods with the right few fats (poly-unsaturated fats and reach in n-3 isomers) and with a low glycemic index; we could privilege raw food instead of complex fat-sweet dishes, whose equivalence is: little amount = high calories.
In this last case, I would doubt you would ever reach immediately the satiety feeling.
Foods reach in Omega-3
Flaxseeds
| Rainbow Trout
| Cauliflower
|
|---|---|---|
Walnuts
| Fresh tuna
| Ground cloves
|
Salmon
| Flaxseed Oil
| Broccoli
|
Herring
| Canned Tuna
| Kale
|
Sardines
| Edamame
| Cabbage
|
Scallops
| Tofu
| |
Mackerel
| Squash
|
Right fats
Right fats? It seems a joke, but it is not! What are right fats and why they are so?
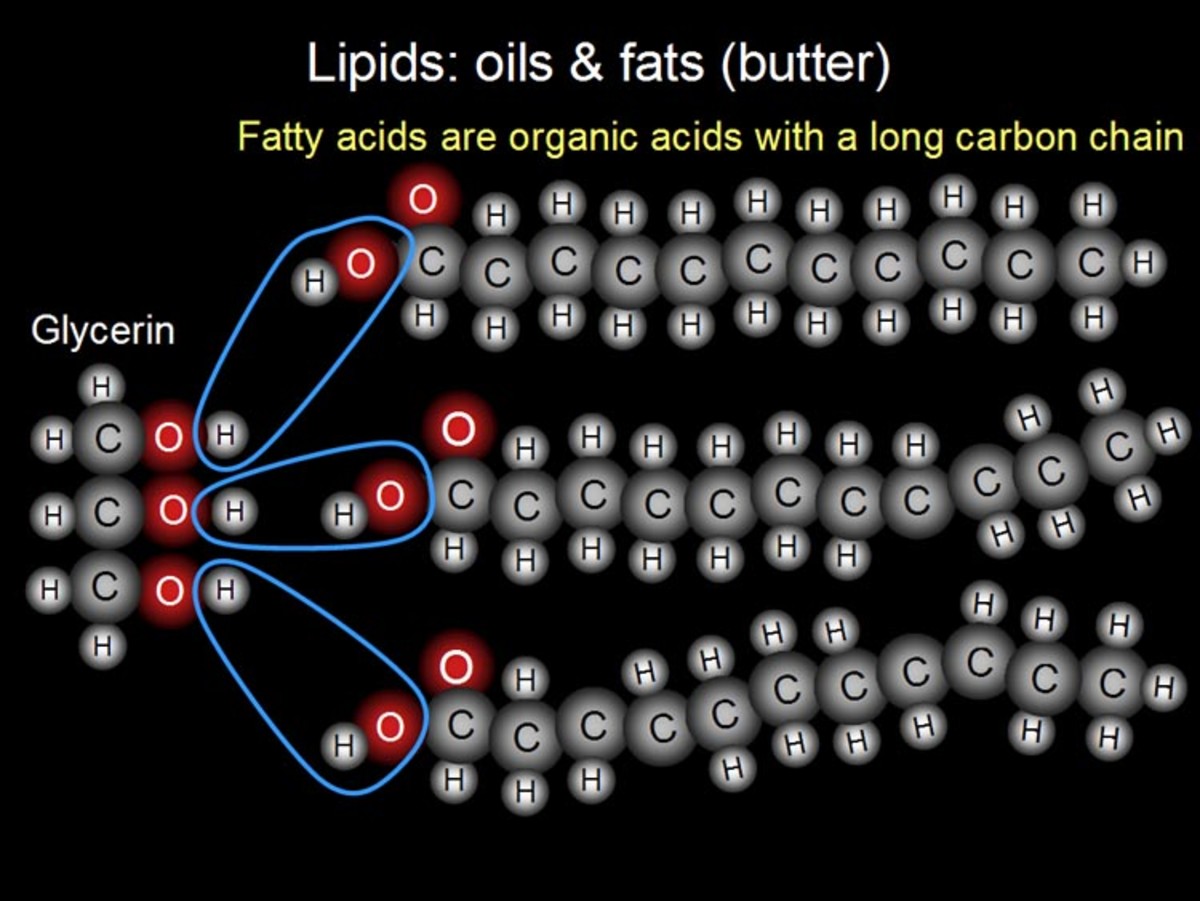
Fats are composed of lipids. These are organic compounds formed by a chain of hydrocarbon atoms and a carboxylic group in one end that confers them the acidic property.
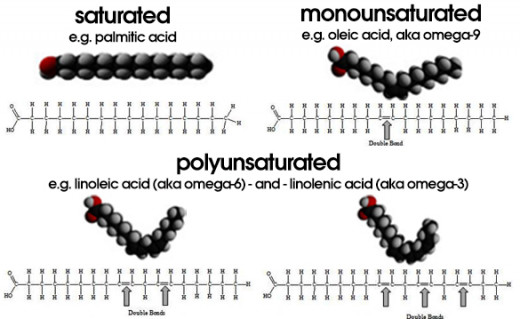
Lipids are distinguished in saturated and unsaturated on the base of the structure of their chain
Monounsaturated fatty acids ('MUFAs', or more plainly monounsaturated fats) are fatty acids that have one double bond in the fatty acid chain with all of the remainder carbon atoms being single-bonded. By contrast, polyunsaturated fatty acids (PUFAs) have more than one double bond.
Fatty acids are long-chained molecules having an alkyl group at one end and a carboxylic acid group at the other end. Fatty acid viscosity (thickness) and melting temperature increases with decreasing number of double bonds; therefore, monounsaturated fatty acids have a higher melting point than polyunsaturated fatty acids (more double bonds) and a lower melting point than saturated fatty acids (no double bonds). Monounsaturated fatty acids are liquids at room temperature and semisolid or solid when refrigerated.
Polyunsaturated fats are triglycerides in which the hydrocarbon tails constitutes polyunsaturated fatty acids (PUFA) (fatty acids possessing more than a single carbon–carbon double bond). Polyunsaturated fat can be found mostly in nuts, seeds, fish, algae, leafy greens, and krill. "Unsaturated" refers to the fact that the molecules contain less than the maximum amount of hydrogen. These materials exist as cis or trans isomers depending on the geometry of the double bond.
The position of the carbon-carbon double bonds in carboxylic acid chains in fats is designated by Greek letters. The carbon atom closest to the carboxyl group is the alpha carbon, the next carbon is the beta carbon and so on. In fatty acids the carbon atom of the methyl group at the end of the hydrocarbon chain is called the omega carbon because omega is the last letter of the Greek alphabet. Omega-3 fatty acids have double bond three carbons away from the methyl carbon, whereas omega-6 fatty acids have a double bond six carbons away from the methyl carbon.
The chain, in virtue of his composition (presence of double bonds between the individual carbon atoms), assumes a deviated direction from the main axis (in the cis configuration; not in the trans configuration) of the molecule. When lipids, hydrophobic, aggregate to form different macromolecules, the deviation from their main axis avoid the formation of a compact structure with the result of a more fluid and malleable macromolecule (this is what distinguishes fluid oils from solid fats). When fluid oils are frozen, they crystallize in a more thermodynamically stable form that confers them a solid state.
Saturated fat is fat that consists of triglycerides containing only saturated fatty acids. Saturated fatty acids have no double bonds between the individual carbon atoms of the fatty acid chain. That is, the chain of carbon atoms is fully "saturated" with hydrogen atoms. There are many kinds of naturally occurring saturated fatty acids, which differ mainly in number of carbon atoms, from 3 carbons (propionic acid) to 36 (hexatriacontanoic acid).
Various fats contain different proportions of saturated and unsaturated fat. Examples of foods containing a high proportion of saturated fat include animal fat products such as cream, cheese, butter, ghee, suet, tallow, lard, and fatty meats. Certain vegetable products have high saturated fat content, such as coconut oil, cottonseed oil, palm kernel oil and chocolate. Many prepared foods are high in saturated fat content, such as pizza, dairy desserts, bacon and sausage.
The consumption of saturated fat is generally considered a risk factor for dyslipidemia, which in turn is a risk factor for some types of cardiovascular disease.
There are strong, consistent, and graded relationships between saturated fat intake, blood cholesterol levels, and the mass occurrence of cardiovascular disease. The relationships are accepted as causal. Abnormal blood lipid levels, that is high total cholesterol, high levels of triglycerides, high levels of low-density lipoprotein (LDL, "bad" cholesterol) or low levels of high-density lipoprotein (HDL, "good" cholesterol) cholesterol are all associated with increased risk of heart disease and stroke.
Meta-analyses have found a significant relationship between saturated fat and serum cholesterol levels. High total cholesterol levels, which may be caused by many factors, are associated with an increased risk of cardiovascular disease. However, other indicators measuring cholesterol such as high total/HDL cholesterol ratio are more predictive than total serum cholesterol. In a study of myocardial infarction in 52 countries, the ApoB/ApoA1 (related to LDL and HDL, respectively) ratio was the strongest predictor of CVD among all risk factors. There are other pathways involving obesity, triglyceride levels, insulin sensitivity, endothelial function, and thrombogenicity, among others, that play a role in CVD, although it seems, in the absence of an adverse blood lipid profile, the other known risk factors have only a weak atherogenic effect. Different saturated fatty acids have differing effects on various lipid levels.
Other diseases are associated with a diet reach in saturated lipids
In nature, fatty acids generally have cis (as opposed to trans) configurations. In food production, liquid cis-unsaturated fats such as vegetable oils are hydrogenated to produce saturated fats, which have more desirable physical properties e.g. they melt at a desirable temperature (30–40 °C). Trans fats are a contaminant introduced by a side reaction on the catalyst in partial hydrogenation.
Although trans fats are edible, consumption of trans fats has shown to increase the risk of coronary heart disease in part by raising levels of the lipoprotein LDL (so-called "bad cholesterol"), lowering levels of the lipoprotein HDL ("good cholesterol"), increasing triglycerides in the bloodstream and promoting systemic inflammation.
We consider unsaturated fats as good fats for many reasons: they contain “good lipids” present in many cellular structures (membranes and organelles; they contribute to their fluidity); they contain less lipids than solid fats and consequently less calories and are easily digested. Another virtue of unsaturated fats is that they can substitute and eliminate saturated fats. Some unsaturated lipids are particularly important. The so called omega-3 (n-3) and -6 (n-6) lipids have some anti-peroxidative properties (in particular docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA)) with a cardiac and cerebral protection.
Common sources of animal omega-3 EPA and DHA fatty acids include fish oils, egg oil, squid oils, krill oil, while some plant oils contain the omega 3 ALA fatty acid such as walnut, seabuckthorn and chia seeds, along with berry oils, clary sage seed oil, algal oil, flaxseed oil, Sacha Inchi oil, Echium oil, and hemp oil.
After this brief presentation, you should know why selecting the right fatty food is important.
Carbohydrate
A carbohydrate consists of carbon (C), hydrogen (H), and oxygen (O) atoms with the empirical formula Cm(H2O)n (where m could be different from n). Carbohydrates are technically hydrates of carbon; structurally it is more accurate to view them as polyhydroxy aldehydes and ketones. In biochemistry it is a synonym of saccharide. The carbohydrates (saccharides) are divided into four chemical groups: monosaccharides, disaccharides, oligosaccharides, and polysaccharides. In general, the monosaccharides and disaccharides, which are smaller (lower molecular weight) carbohydrates, are commonly referred to as sugars. They are easily absorbed in by the bowel.
Polysaccharides serve for the storage of energy (e.g., starch and glycogen), and as structural components (e.g., cellulose in plants and chitin in arthropods). The 5-carbon monosaccharide ribose is an important component of coenzymes (e.g., ATP, FAD, and NAD) and the backbone of the genetic molecule known as RNA. The related deoxyribose is a component of DNA. Saccharides and their derivatives include many other important biomolecules that play key roles in the immune system, fertilization, preventing pathogenesis, blood clotting, and development.
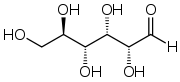
Natural saccharides are generally built of simple carbohydrates called monosaccharides with general formula (CH2O)n where n is three or more. A typical monosaccharide has the structure H-(CHOH)x(C=O)-(CHOH)y-H, that is, an aldehyde or ketone with many hydroxyl groups added, usually one on each carbon atom that is not part of the aldehyde or ketone functional group. Examples of monosaccharides are glucose, fructose, and glyceraldehydes.
The open-chain form of a monosaccharide often coexists with a closed ring form where the aldehyde/ketone carbonyl group carbon (C=O) and hydroxyl group (-OH) react forming a hemiacetal with a new C-O-C bridge.
Monosaccharides can be linked together into what are called polysaccharides (or oligosaccharides) in a large variety of ways. Many carbohydrates contain one or more modified monosaccharide units that have had one or more groups replaced or removed. For example, deoxyribose, a component of DNA, is a modified version of ribose; chitin is composed of repeating units of N-acetyl glucosamine, a nitrogen-containing form of glucose.
Monosaccharides are classified according to three different characteristics: the placement of its carbonyl group, the number of carbon atoms it contains, and its chiral handedness. If the carbonyl group is an aldehyde, the monosaccharide is an aldose; if the carbonyl group is a ketone, the monosaccharide is a ketose. Monosaccharides with three carbon atoms are called trioses, those with four are called tetroses, five are called pentoses, six are hexoses, and so on. These two systems of classification are often combined. For example, glucose is an aldohexose (a six-carbon aldehyde), ribose is an aldopentose (a five-carbon aldehyde), and fructose is a ketohexose (a six-carbon ketone).
Each carbon atom bearing a hydroxyl group (-OH), with the exception of the first and last carbons, are asymmetric, making them stereo centers with two possible configurations each (R or S). Because of this asymmetry, a number of isomers may exist for any given monosaccharide formula. Using Le Bel-van't Hoff rule, the aldohexose D-glucose, for example, has the formula (C·H2O) 6, of which four of its six carbons atoms are stereogenic, making D-glucose one of 24=16 possible stereoisomers. The assignment of D or L is made according to the orientation of the asymmetric carbon furthest from the carbonyl group: in a standard Fischer projection if the hydroxyl group is on the right the molecule is a D sugar, otherwise it is an L sugar. The "D-" and "L-" prefixes should not be confused with "d-" or "l-", which indicate the direction that the sugar rotates plane polarized light. This usage of "d-" and "l-" is no longer followed in carbohydrate chemistry.
The aldehyde or ketone group of a straight-chain monosaccharide will react reversibly with a hydroxyl group on a different carbon atom to form a hemiacetal or hemiketal, forming a heterocyclic ring with an oxygen bridge between two carbon atoms. Rings with five and six atoms are called furanose and pyranose forms, respectively, and exist in equilibrium with the straight-chain form.
During the conversion from straight-chain form to the cyclic form, the carbon atom containing the carbonyl oxygen, called the anomeric carbon, becomes a stereogenic center with two possible configurations: The oxygen atom may take a position either above or below the plane of the ring. The resulting possible pair of stereoisomers is called anomers. In the α anomer, the -OH substituent on the anomeric carbon rests on the opposite side (trans) of the ring from the CH2OH side branch. The alternative form, in which the CH2OH substituent and the anomeric hydroxyl are on the same side (cis) of the plane of the ring, is called the β anomer.
Monosaccharides are the major source of fuel for metabolism, being used both as an energy source (glucose being the most important in nature) and in biosynthesis. When monosaccharides are not immediately needed by many cells they are often converted to more space-efficient forms, often polysaccharides. In many animals, including humans, this storage form is glycogen, especially in liver and muscle cells. In plants, starch is used for the same purpose. The most abundant carbohydrate, cellulose, is a structural component of the cell wall of plants and many forms of algae. Ribose is a component of RNA. Deoxyribose is a component of DNA. Lyxose is a component of lyxoflavin found in human heart. Ribulose and xylulose occurs in the pentose phosphate pathway. Galactose, a component of milk sugar lactose, is found in galactolipids in plant cell membranes and in glycoproteins in many tissues. Mannose occurs in human metabolism, especially in the glycosylation of certain proteins. Fructose, or fruit sugar, is found in many plants and in humans, it is metabolized in the liver, absorbed directly into the intestines during digestion, and found in semen. Trehalose, a major sugar of insects, is rapidly hydrolyzed into two glucose molecules to support continuous flight.
Two joined monosaccharides are called a disaccharide and these are the simplest polysaccharides. Examples include sucrose and lactose. They are composed of two monosaccharide units bound together by a covalent bond known as a glycosidic linkage formed via a dehydration reaction, resulting in the loss of a hydrogen atom from one monosaccharide and a hydroxyl group from the other. The formula of unmodified disaccharides is C12H22O11. Although there are numerous kinds of disaccharides, a handful of disaccharides are particularly notable.
Sucrose, is the most abundant disaccharide, and the main form in which carbohydrates are transported in plants. It is composed of one D-glucose molecule and one D-fructose molecule.
Lactose, a disaccharide composed of one D-galactose molecule and one D-glucose molecule, occurs naturally in mammalian milk. Other notable disaccharides include maltose (two D-glucoses linked α-1,4) and cellulobiose (two D-glucoses linked β-1,4). Disaccharides can be classified into two types. They are reducing and non-reducing disaccharides. If the functional group is present in bonding with another sugar unit, it is called a reducing disaccharide or biose.
Nutrition
Carbohydrate consumed in food yields 3.87 calories of energy per gram for simple sugars, and 3.57 to 4.12 calories per gram for complex carbohydrate in most other foods. Foods with high carbohydrate are often highly processed or refined foods made from plants, including sweets, cookies and candy, table sugar, honey, soft drinks, breads and crackers, jams and fruit products, pastas and breakfast cereals. Unrefined foods usually contain lower amounts of carbohydrate, including beans, tubers, rice, and unrefined fruit. Foods from animal carcass have the lowest carbohydrate, but milk does contain lactose.
Carbohydrates are a common source of energy in living organisms; however, no carbohydrate is an essential nutrient in humans. Humans are able to obtain most of their energy requirement from protein and fats, though the potential for some negative health effects of extreme carbohydrate restriction remains, as the issue has not been studied extensively so far. However, in the case of dietary fiber – indigestible carbohydrates which are not a source of energy – inadequate intake can lead to significant increases in mortality.
Following a diet consisting of very low amounts of daily carbohydrate for several days will usually result in higher levels of blood ketone bodies than an isocaloric diet with similar protein content. This relatively high level of ketone bodies is commonly known as ketosis and is very often confused with the potentially fatal condition often seen in type 1 diabetics known as diabetic ketoacidosis. Somebody suffering ketoacidosis will have much higher levels of blood ketone bodies along with high blood sugar, dehydration and electrolyte imbalance.
Long-chain fatty acids cannot cross the blood–brain barrier, but the liver can break these down to produce ketones. However the medium-chain fatty acids octanoic and heptanoic acids can cross the barrier and be used by the brain, which normally relies upon glucose for its energy. Gluconeogenesis allows humans to synthesize some glucose from specific amino acids: from the glycerol backbone in triglycerides and in some cases from fatty acids.
Glucose is a nearly universal and accessible source of calories. Polysaccharides are also common sources of energy. Many organisms can easily break down starches into glucose, however, most organisms cannot metabolize cellulose or other polysaccharides like chitin and arabinoxylans.
Based on the effects on risk of heart disease and obesity, the Institute of Medicine recommends that American and Canadian adults get between 45–65% of dietary energy from carbohydrates. The Food and Agriculture Organization and World Health Organization jointly recommend that national dietary guidelines set a goal of 55–75% of total energy from carbohydrates, but only 10% directly from sugars (their term for simple carbohydrates).
Catabolism is the metabolic reaction which cells undergo to extract energy. There are two major metabolic pathways of monosaccharide catabolism: glycolysis and the citric acid cycle.
In glycolysis, oligo/polysaccharides are cleaved first to smaller monosaccharides by enzymes called glycoside hydrolases. The monosaccharide units can then enter into monosaccharide catabolism. In some cases, as with humans, not all carbohydrate types are usable as the digestive and metabolic enzymes necessary are not present.
Insulin
In mammals, insulin is synthesized in the pancreas within the β-cells of the islets of Langerhans. One million to three million islets of Langerhans (pancreatic islets) form the endocrine part of the pancreas, which is primarily an exocrine gland. The endocrine portion accounts for only 2% of the total mass of the pancreas. Within the islets of Langerhans, beta cells constitute 65–80% of all the cells.
Insulin consists of two polypeptide chains, the A- and B- chains, linked together by disulfide bonds. It is however first synthesized as a single polypeptide called preproinsulin in pancreatic β-cells. Preproinsulin contains a 24-residue signal peptide which directs the nascent polypeptide chain to the rough endoplasmic reticulum (RER). The signal peptide is cleaved as the polypeptide is translocated into lumen of the RER, forming proinsulin. In the RER the proinsulin folds into the correct conformation and 3 disulfide bonds are formed. About 5–10 min after its assembly in the endoplasmic reticulum, proinsulin is transported to the trans-Golgi network (TGN) where immature granules are formed. Transport to the TGN may take about 30 min.
Proinsulin undergoes maturation into active insulin through the action of cellular endopeptidases known as prohormone convertases (PC1 and PC2), as well as the exoprotease carboxypeptidase E. The endopeptidases cleave at 2 positions, releasing a fragment called the C-peptide, and leaving 2 peptide chains, the B- and A- chains, linked by 2 disulfide bonds. The cleavage sites are each located after a pair of basic residues (lysine-64 and arginine-65, and arginine-31 and -32). After cleavage of the C-peptide, these 2 pairs of basic residues are removed by the carboxypeptidase. The C-peptide is the central portion of proinsulin, and the primary sequence of proinsulin goes in the order "B-C-A" (the B and A chains were identified on the basis of mass and the C-peptide was discovered later).
The resulting mature insulin is packaged inside mature granules waiting for metabolic signals (such as leucine, arginine, glucose and mannose) and vagal nerve stimulation to be exocytosed from the cell into the circulation.
The endogenous production of insulin is regulated in several steps along the synthesis pathway:
- At transcription from the insulin gene
- In mRNA stability
- At the mRNA translation
- In the posttranslational modifications
Beta cells in the islets of Langerhans release insulin in two phases. The first phase release is rapidly triggered in response to increased blood glucose levels. The second phase is a sustained, slow release of newly formed vesicles triggered independently of sugar. The description of first phase release is as follows:
- Glucose enters the β-cells through the glucose transporter, GLUT2.
- Glucose goes into glycolysis and the Krebs cycle, where multiple, high-energy ATP molecules are produced by oxidation, leading to a rise in the ATP:ADP ratio within the cell.
- An increased intracellular ATP:ADP ratio closes the ATP-sensitive SUR1/Kir6.2 potassium channel. This prevents potassium ions (K+) from leaving the cell by facilitated diffusion, leading to a buildup of potassium ions. As a result, the inside of the cell becomes more positive with respect to the outside, leading to the depolarization of the cell surface membrane.
- On depolarization, voltage-gated calcium ion (Ca2+) channels open which allows calcium ions to move into the cells by facilitated diffusion.
- An increased intracellular calcium ion concentration causes the activation of phospholipase C, which cleaves the membrane phospholipid phosphatidyl inositol 4,5-bisphosphate into inositol 1,4,5-trisphosphate and diacylglycerol.
- Inositol 1,4,5-trisphosphate (IP3) binds to receptor proteins in the plasma membrane of the endoplasmic reticulum (ER). This allows the release of Ca2+ ions from the ER via IP3-gated channels, and further raises the intracellular concentration of calcium ions.
- Significantly increased amounts of calcium ions in the cells cause the release of previously synthesized insulin, which has been stored in secretory vesicles.
This is the primary mechanism for release of insulin. Other substances known to stimulate insulin release include the amino acids arginine and leucine, parasympathetic release of acetylcholine (via phospholipase C), sulfonylurea, cholecystokinin (CCK, via phospholipase C), and the gastrointestinally derived incretins glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP).
Release of insulin is strongly inhibited by the stress hormone norepinephrine (noradrenaline), which leads to increased blood glucose levels during stress. It appears that release of catecholamines by the sympathetic nervous system has conflicting influences on insulin release by beta cells, because insulin release is inhibited by α2-adrenergic receptors and stimulated by β2-adrenergic receptors. The net effect of norepinephrine from sympathetic nerves and epinephrine from adrenal glands on insulin release is inhibition due to dominance of the α-adrenergic receptors.
When the glucose level comes down to the usual physiologic value, insulin release from the β-cells slows or stops. If blood glucose levels drop lower than this, especially to dangerously low levels, release of hyperglycemic hormones (most prominently glucagon from islet of Langerhans alpha cells) forces release of glucose into the blood from cellular stores, primarily liver cell stores of glycogen. By increasing blood glucose, the hyperglycemic hormones prevent or correct life-threatening hypoglycemia.
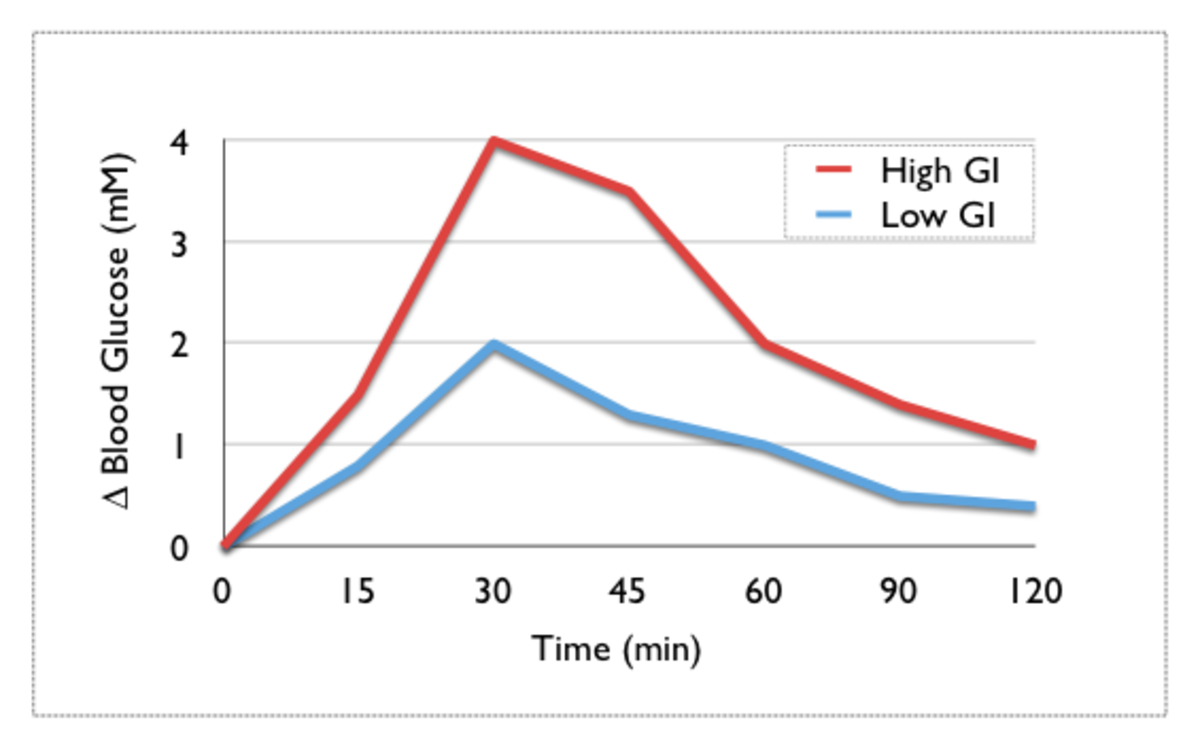
Evidence of impaired first-phase insulin release can be seen in the glucose tolerance test, demonstrated by a substantially elevated blood glucose level at 30 minutes, a marked drop by 60 minutes, and a steady climb back to baseline levels over the following hourly time points.
The greater the rate of increase in your blood sugar, the more chance that your body will release an excess amount of insulin, and drive your blood sugar back down too low.
Therefore, when you eat foods that cause a large and rapid glycemic response, you may feel an initial elevation in energy and mood as your blood sugar rises, but this is followed by a cycle of increased fat storage, lethargy, and more hunger!
Although increased fat storage may sound bad enough, individuals with diabetes (diabetes mellitus, types 1 and 2) have an even worse problem. Their bodies’ inability to secrete or process insulin causes their blood sugar to raise too high, leading to a host of additional medical problems.
Two types of tissues are most strongly influenced by insulin, as far as the stimulation of glucose uptake is concerned: muscle cells (myocytes) and fat cells (adipocytes). The former are important because of their central role in movement, breathing, circulation, etc., and the latter because they accumulate excess food energy against future needs. Together, they account for about two-thirds of all cells in a typical human body.
Insulin binds to the extracellular portion of the alpha subunits of the insulin receptor. This, in turn, causes a conformational change in the insulin receptor that activates the kinase domain residing on the intracellular portion of the beta subunits. The activated kinase domain autophosphorylates tyrosine residues on the C-terminus of the receptor as well as tyrosine residues in the IRS-1 protein.
- phosphorylated IRS-1, in turn, binds to and activates phosphoinositol 3 kinase (PI3K)
- PI3K catalyzes the reaction PIP2 + ATP → PIP3 + ADP
- PIP3 activates protein kinase B (PKB)
- PKB phosphorylates glycogen synthase kinase (GSK) and thereby inactivates GSK
- GSK can no longer phosphorylate glycogen synthase (GS)
- unphosphorylated GS makes more glycogen
- PKB also facilitates vesicle fusion, resulting in an increase in GLUT4 transporters in the plasma membrane
After the signal has been produced, termination of signaling is then needed. As mentioned below in the section on degradation, endocytosis and degradation of the receptor bound to insulin is a main mechanism to end signaling. In addition, signaling can be terminated by dephosphorylating the tyrosine residues by tyrosine phosphatases. Serine/Threonine kinases are also known to reduce the activity of insulin. Finally, with insulin action being associated with the number of receptors on the plasma membrane, a decrease in the amount of receptors also leads to termination of insulin signaling.
The actions of insulin (indirect and direct) on cells are many; all are important. In particular we will consider here few of those functions:
- Increased glycogen synthesis – insulin forces storage of glucose in liver (and muscle) cells in the form of glycogen; lowered levels of insulin cause liver cells to convert glycogen to glucose and excrete it into the blood. This is the clinical action of insulin, which is directly useful in reducing high blood glucose levels as in diabetes.
- Increased lipid synthesis – insulin forces fat cells to take in blood lipids, which are converted to triglycerides; lack of insulin causes the reverse.
- Increased esterification of fatty acids – forces adipose tissue to make fats (i.e., triglycerides) from fatty acid esters; lack of insulin causes the reverse.
- Decreased lipolysis – forces reduction in conversion of fat cell lipid stores into blood fatty acids; lack of insulin causes the reverse.
- Decreased autophagy - decreased level of degradation of damaged organelles. Postprandial levels inhibit autophagy completely.
- Increased amino acid uptake – forces cells to absorb circulating amino acids; lack of insulin inhibits absorption.
- Arterial muscle tone – forces arterial wall muscle to relax, increasing blood flow, especially in microarteries; lack of insulin reduces flow by allowing these muscles to contract.
- Increase in the secretion of hydrochloric acid by parietal cells in the stomach
- Decreased renal sodium excretion.
Insulin also influences other body functions, such as vascular compliance and cognition. Once insulin enters the human brain, it enhances learning and memory and benefits verbal memory in particular.
Insulin disturbance
Diabetes mellitus – general term referring to all states characterized by hyperglycemia
Type 1 – autoimmune-mediated destruction of insulin-producing β-cells in the pancreas, resulting in absolute insulin deficiency
Type 2 – multifactoral syndrome with combined influence of genetic susceptibility and influence of environmental factors, the best known being obesity, age, and physical inactivity, resulting in insulin resistance in cells requiring insulin for glucose absorption.
Insulinoma - a tumor of pancreatic β-cells producing excess insulin or reactive hypoglycemia.
Metabolic syndrome – a poorly understood condition first called Syndrome X by Gerald Reaven, Reaven's Syndrome after Reaven, CHAOS in Australia (from the signs that seem to travel together). It is currently not clear whether these signs have a single, treatable cause, or are the result of body changes leading to type 2 diabetes. It is characterized by elevated blood pressure, dyslipidemia (disturbances in blood cholesterol forms and other blood lipids), and increased waist circumference (at least in populations in much of the developed world). The basic underlying cause may be the insulin resistance that precedes type 2 diabetes, which is a diminished capacity for insulin response in some tissues (e.g., muscle, fat). It is common that morbidities, such as essential hypertension, obesity, type 2 diabetes, and cardiovascular disease (CVD) develop.
Polycystic ovary syndrome – a complex syndrome in women in the reproductive years where anovulation and androgen excess are commonly displayed as hirsutism. In many cases of PCOS, insulin resistance is present.
Artificial sweeteners
Today we have artificial sweeteners, (stevia, aspartame, sucralose, neotame, acesulfame potassium, and saccharin); so let us use less sugar. The majority of sugar substitutes approved for food use are artificially synthesized compounds. However, some bulk natural sugar substitutes are known, including sorbitol and xylitol, which are found in berries, fruit, vegetables, and mushrooms. It is not commercially viable to extract these products from fruits and vegetables, so they are produced by catalytic hydrogenation of the appropriate reducing sugar. For example, xylose is converted to xylitol, lactose to lactitol, and glucose to sorbitol. Other natural substitutes are known but are yet to gain official approval for food use.
Some non-sugar sweeteners are polyols, also known as "sugar alcohols". These are, in general, less sweet than sucrose but have similar bulk properties and can be used in a wide range of food products. Sometimes the sweetness profile is 'fine-tuned' by mixing with high-intensity sweeteners. As with all food products, the development of a formulation to replace sucrose is a complex proprietary process.
Benefits of sweeteners are many:
- Contribute to cut the glycemic index of a diet (for controlling the diabetes or the weight), food or dish;
- Prevent the cariogenic effect being not fermented by the microflora of the dental plaque. Xylitol works to prevent bacteria from adhering to the tooth surface, thus preventing plaque formation and eventually decay. Normal sugar feed the oral bacterial flora increasing their pathological power.
- Reactive hypoglycemia produces an excess of insulin after quickly absorbing glucose into the blood stream. This causes their blood glucose levels to fall below the amount needed for proper body and brain function. As a result, like diabetics, intake of high-glycemic foods like white bread must be avoided, and often choose artificial sweeteners as an alternative.
- Individuals may opt to substitute refined white sugar with less-processed sugars, such as fruit juice or maple syrup.
- Many sugar substitutes are cheaper than sugar.
The theory behind the Glycemic Index is simply to minimize insulin-related problems by identifying and avoiding foods that have the greatest effect on your blood sugar.
The effects that different foods have on blood glucose levels (i.e., blood sugar) vary considerably. The glycemic index or glycemic index (GI) attempts to measure this variation. It does so by estimating how much each gram of available carbohydrate (total carbohydrate minus fiber) in a food raises a person's blood glucose level following consumption of that food, relative to consumption of pure glucose (the defining standard), which has a glycemic index of 100. Because a large increase in the glucose response to a food typically makes for a steeper initial climb in that response, it is also a rough measure of how quickly blood glucose levels may rise after eating a particular food, though this rapidity can be influenced by the quantity of fat eaten with the food, as well as by other factors.
Nutritionists used to believe that all simple sugars digested quickly, caused a rapid rise in blood sugar, and that the opposite was true for "complex carbohydrates". But that's not always the case. While many sweet and sugary foods do have high GI's, some starchy foods like potatoes or white bread score even higher than honey or table sugar (sucrose)!
How Glycemic Load Improves the Glycemic Index
The glycemic index (GI) and glycemic load concepts have been developed to characterize food behavior during human digestion. They rank carbohydrate-rich foods based on the rapidity and magnitude of their effect on blood glucose levels. Glycemic index is a measure of how quickly food glucose is absorbed, while glycemic load is a measure of the total absorbable glucose in foods. The insulin index is a similar, more recent classification method that ranks foods based on their effects on blood insulin levels, which are caused by glucose (or starch) and some amino acids in food.
Although most candy has a relatively high Glycemic Index, eating a single piece of candy will result in a relatively small glycemic response. Why? Well, simply because your body's glycemic response is dependent on both the type AND the amount of carbohydrate consumed. This concept, known as Glycemic Load, was first popularized in 1997 by Dr. Walter Willett and associates at the Harvard School of Public Health. Glycemic Load is calculated this way:
GL = GI/100 x Net Carbs
(Net Carbs are equal to the Total Carbohydrates minus Dietary Fiber)
Therefore, you can control your glycemic response by consuming low-GI foods and/or by restricting your intake of carbohydrates.
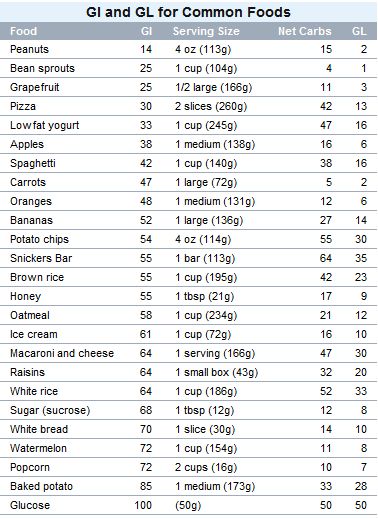
The table shows values of the Glycemic Index (GI) and Glycemic Load (GL) for a few common foods. GI's of 55 or below are considered low, and 70 or above are considered high. GL's of 10 or below are considered low, and 20 or above are considered high.
Some proponents of the Glycemic Index (including many diet books authors) would like you to believe that GI and GL are all that matters when selecting which foods to eat. In reality, diet is a more complex issue than that. ND agrees that the Glycemic Index is a marvelous tool for ranking carbohydrates (and much better than the old "simple" and "complex carbohydrate" designations).
How much should I eat to preserve my weight with few fats and carbohydrates?
Other data could have been presented here to deeply understand the GI and GL and the importance of the carbohydrate and fat control in our diet.
Definitely, a low caloric density is equivalent to a higher volume of the food we can consume to balance the amount of calories in our diet.
There was a good correlation between the Satiety Index values and each food's Caloric density. There were also significant but lesser correlations between the index and each food's levels of net carbohydrates, fat, dietary fiber, and protein. From the mathematical model developed, ND was able to create an equation to convert a food's nutrient profile into a predicted satiety index, which we call the Fullness Factor™.
The Fullness Factor™ has been normalized so that all resultant values fall into a range of 0 to 5. The calculated Fullness Factor for white bread is 1.8, so values above 1.8 indicate foods that are likely to be more satiating than white bread, and values less than 1.8 indicate foods that are likely to be less satiating. A food's Fullness Factor is independent of its serving size.
We already have analyzed the Fullness Factor™ (FF). Because of the strong relation between satiety and a food's weight, some researchers have recommended the consumption of foods with low Caloric densities - i.e. foods that have the fewest total Calories per gram. One of the most notable of these researchers is nutritionist Barbara Rolls, Ph.D., whose prior best-selling diet book, Volumetrics, explained her use of low Caloric density foods for weight loss. A more recent diet that makes use of low Caloric density foods is the Negative Calorie Diet. There are also many specialty diets that use a low Caloric density approach. Included among these are the cabbage soup diet and the grapefruit diet.
In summary, if you had been able to reduce refined sugar and in general carbohydrates (caloric desserts) in your diet, and to avoid the use of vegetable oils (or animals’ fats), you can eat more (different foods, mainly raw foods), feel the satiety after each meal, lose weight or just preserve it with a more safe diet for your body.
I have to eat continuously…! The volume of food doubled or triplicated without bad fat and refined sugar…